当前位置:
X-MOL 学术
›
BBA Gen. Subj.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The intrinsic stability of H2B-ubiquitylated nucleosomes and their in vitro assembly/disassembly by histone chaperone NAP1.
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2019-11-27 , DOI: 10.1016/j.bbagen.2019.129497 Wladyslaw A Krajewski 1
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2019-11-27 , DOI: 10.1016/j.bbagen.2019.129497 Wladyslaw A Krajewski 1
Affiliation
|
BACKGROUND
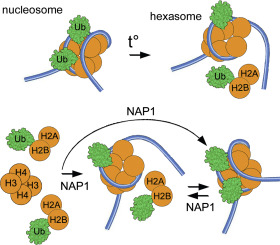
Apart the gene-regulatory functions as docking sites for histone 'readers', some histone modifications could directly affect nucleosome structure. The H2BK34-ubiquitylation deposited by MOF-MSL complex, increases nucleosome dynamics in vitro and promotes donation of one H2A/H2B dimer to histone acceptors.
METHODS
We evaluated temperature-depended stability of H2BK34-ubiquitylated nucleosomes under 'physiological' ionic conditions in the presence or absence of histone acceptor, and examined assembly and disassembly of ubiquitylated nucleosomes in vitro by recombinant mouse NAP1.
RESULTS
H2BK34ub modification is sufficient to promote selective eviction of only one H2A/H2B dimer independently of histone-binding agents. Despite the robust H2A/H2B dimer-displacement effect of mNAP1 with the H2BK34ub (but not unmodified) nucleosomes, NAP1 could assemble symmetrically- or asymmetrically ubiquitylated nucleosomes under 'physiological' conditions in vitro.
CONCLUSIONS AND GENERAL SIGNIFICANCE
The increased mobility of one nucleosomal H2A/H2B dimer is an intrinsic nucleosome destabilizing property of H2BK34 ubiquitylation that has the intranucleosome bases. The ability of NAP to reasonably efficiently assemble H2BK34-ubiquitylated nucleosomes supposes a potential mechanism for deposition/distribution of H2BK34ub mark in the MOF-MSL independent manner (for example, during histone dimer exchange upon transcription elongation).
中文翻译:

H2B泛素化核小体的固有稳定性及其在体外的组蛋白伴侣NAP1组装/拆卸。
背景技术除了基因调节功能用作组蛋白“阅读者”的停靠位点外,一些组蛋白修饰还可以直接影响核小体的结构。由MOF-MSL复合物沉积的H2BK34泛素化,增加了体外的核小体动力学,并促进了一个H2A / H2B二聚体向组蛋白受体的捐赠。方法我们评估了在存在或不存在组蛋白受体的“生理”离子条件下H2BK34泛素化核小体的温度依赖性稳定性,并通过重组小鼠NAP1在体外检查了泛素化核小体的组装和拆卸。结果H2BK34ub修饰足以促进仅驱逐一个H2A / H2B二聚体,而与组蛋白结合剂无关。尽管mNAP1与H2BK34ub(但不是未修饰的)核小体具有强大的H2A / H2B二聚体置换作用,但NAP1可以在体外“生理”条件下组装对称或不对称的泛素化核小体。结论和一般意义一个核小体H2A / H2B二聚体的增加的迁移性是具有核小体内碱基的H2BK34泛素化的固有核小体去稳定特性。NAP合理有效地组装H2BK34-泛素化核小体的能力为MOF-MSL独立方式(例如,在转录延长时的组蛋白二聚体交换期间)沉积/分布H2BK34ub标记的潜在机制提供了可能。结论和一般意义一个核小体H2A / H2B二聚体的增加的迁移性是具有核小体内碱基的H2BK34泛素化的固有核小体去稳定特性。NAP合理有效地组装H2BK34-泛素化的核小体的能力假定了MOF-MSL独立方式(例如,在转录延长时的组蛋白二聚体交换期间)H2BK34ub标记沉积/分布的潜在机制。结论和一般意义一个核小体H2A / H2B二聚体的增加的迁移性是具有核小体内碱基的H2BK34泛素化的固有核小体去稳定特性。NAP合理有效地组装H2BK34-泛素化的核小体的能力假定了MOF-MSL独立方式(例如,在转录延长时的组蛋白二聚体交换期间)H2BK34ub标记沉积/分布的潜在机制。
更新日期:2019-11-28
中文翻译:

H2B泛素化核小体的固有稳定性及其在体外的组蛋白伴侣NAP1组装/拆卸。
背景技术除了基因调节功能用作组蛋白“阅读者”的停靠位点外,一些组蛋白修饰还可以直接影响核小体的结构。由MOF-MSL复合物沉积的H2BK34泛素化,增加了体外的核小体动力学,并促进了一个H2A / H2B二聚体向组蛋白受体的捐赠。方法我们评估了在存在或不存在组蛋白受体的“生理”离子条件下H2BK34泛素化核小体的温度依赖性稳定性,并通过重组小鼠NAP1在体外检查了泛素化核小体的组装和拆卸。结果H2BK34ub修饰足以促进仅驱逐一个H2A / H2B二聚体,而与组蛋白结合剂无关。尽管mNAP1与H2BK34ub(但不是未修饰的)核小体具有强大的H2A / H2B二聚体置换作用,但NAP1可以在体外“生理”条件下组装对称或不对称的泛素化核小体。结论和一般意义一个核小体H2A / H2B二聚体的增加的迁移性是具有核小体内碱基的H2BK34泛素化的固有核小体去稳定特性。NAP合理有效地组装H2BK34-泛素化核小体的能力为MOF-MSL独立方式(例如,在转录延长时的组蛋白二聚体交换期间)沉积/分布H2BK34ub标记的潜在机制提供了可能。结论和一般意义一个核小体H2A / H2B二聚体的增加的迁移性是具有核小体内碱基的H2BK34泛素化的固有核小体去稳定特性。NAP合理有效地组装H2BK34-泛素化的核小体的能力假定了MOF-MSL独立方式(例如,在转录延长时的组蛋白二聚体交换期间)H2BK34ub标记沉积/分布的潜在机制。结论和一般意义一个核小体H2A / H2B二聚体的增加的迁移性是具有核小体内碱基的H2BK34泛素化的固有核小体去稳定特性。NAP合理有效地组装H2BK34-泛素化的核小体的能力假定了MOF-MSL独立方式(例如,在转录延长时的组蛋白二聚体交换期间)H2BK34ub标记沉积/分布的潜在机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号