当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Allosteric Modulation of N-Terminally Cleaved, but Not Full Length CCL3 in CCR1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-11-26 , DOI: 10.1021/acsptsci.9b00059 Olav Larsen 1 , Michael Lückmann 2 , Wijnand J. C. van der Velden 1 , Marta Oliva-Santiago 1 , Matjaz Brvar 3 , Trond Ulven 3, 4 , Thomas M. Frimurer 2 , Stefanie Karlshøj 1 , Mette M. Rosenkilde 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-11-26 , DOI: 10.1021/acsptsci.9b00059 Olav Larsen 1 , Michael Lückmann 2 , Wijnand J. C. van der Velden 1 , Marta Oliva-Santiago 1 , Matjaz Brvar 3 , Trond Ulven 3, 4 , Thomas M. Frimurer 2 , Stefanie Karlshøj 1 , Mette M. Rosenkilde 1
Affiliation
|
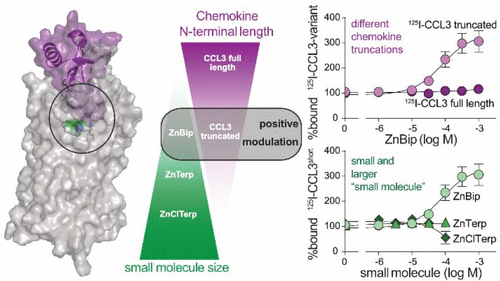
Chemokines undergo post-translational modification such as N-terminal truncations. Here, we describe how N-terminal truncation of full length CCL3(1–70) affects its activity at CCR1. Truncated CCL3(5–70) has 10-fold higher potency and enhanced efficacy in β-arrestin recruitment, but less than 2-fold increased potencies in G protein signaling determined by calcium release, cAMP and IP3 formation. Small positive ago-allosteric ligands modulate the two CCL3 variants differently as the metal ion chelator bipyridine in complex with zinc (ZnBip) enhances the binding of truncated, but not full length CCL3, while a size-increase of the chelator to a chloro-substituted terpyridine (ZnClTerp), eliminates its allosteric, but not agonistic action. By employing a series of receptor mutants and in silico modeling we describe residues of importance for chemokine and small molecule binding. Notably, the chemokine receptor-conserved Glu2877.39 interacts with the N-terminal amine of truncated CCL3(5–70) and with Zn2+ of ZnBip, thereby bridging their binding sites and enabling the positive allosteric effect. Our study emphasizes that small allosteric molecules may act differently toward chemokine variants and thus selectively modulate interactions of specific chemokine subsets with their cognate receptors.
中文翻译:

CCR1中N末端切割但非全长CCL3的选择性变构调节
趋化因子经过翻译后修饰,例如N端截短。在这里,我们描述全长CCL3 (1–70)的N末端截短如何影响其在CCR1的活性。截短的CCL3 (5-70)在β-arrestin募集方面的效力提高了10倍,并且功效增强,但由钙释放,cAMP和IP 3决定的G蛋白信号传导的效力增加了不到2倍编队。较小的阳性前变构配体不同地调节了两个CCL3变体,因为与锌络合的金属离子螯合剂联吡啶增强了截短的CCL3的结合,但不增强全长CCL3的结合,同时螯合剂的尺寸增加了对氯取代的螯合剂的大小。特吡啶(ZnClTerp),消除了其变构作用,但没有激动作用。通过采用一系列受体突变体和计算机模拟,我们描述了对趋化因子和小分子结合重要的残基。值得注意的是,趋化因子受体保守的Glu287 7.39与截短的CCL3 (5-70)的N末端胺以及与Zn 2+相互作用。ZnBip的作用,从而桥接它们的结合位点并实现正构变效应。我们的研究强调,小的变构分子可能对趋化因子变异体有不同的作用,从而选择性地调节特定趋化因子亚群与其同源受体的相互作用。
更新日期:2019-11-28
中文翻译:

CCR1中N末端切割但非全长CCL3的选择性变构调节
趋化因子经过翻译后修饰,例如N端截短。在这里,我们描述全长CCL3 (1–70)的N末端截短如何影响其在CCR1的活性。截短的CCL3 (5-70)在β-arrestin募集方面的效力提高了10倍,并且功效增强,但由钙释放,cAMP和IP 3决定的G蛋白信号传导的效力增加了不到2倍编队。较小的阳性前变构配体不同地调节了两个CCL3变体,因为与锌络合的金属离子螯合剂联吡啶增强了截短的CCL3的结合,但不增强全长CCL3的结合,同时螯合剂的尺寸增加了对氯取代的螯合剂的大小。特吡啶(ZnClTerp),消除了其变构作用,但没有激动作用。通过采用一系列受体突变体和计算机模拟,我们描述了对趋化因子和小分子结合重要的残基。值得注意的是,趋化因子受体保守的Glu287 7.39与截短的CCL3 (5-70)的N末端胺以及与Zn 2+相互作用。ZnBip的作用,从而桥接它们的结合位点并实现正构变效应。我们的研究强调,小的变构分子可能对趋化因子变异体有不同的作用,从而选择性地调节特定趋化因子亚群与其同源受体的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号