当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recent Advances in Four‐Coordinated Planar Cobalt Catalysis in Organic Synthesis
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2019-12-04 , DOI: 10.1002/ajoc.201900625 Takuya Michiyuki 1 , Kimihiro Komeyama 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2019-12-04 , DOI: 10.1002/ajoc.201900625 Takuya Michiyuki 1 , Kimihiro Komeyama 1
Affiliation
|
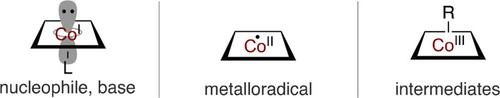
In the field of transition metal‐catalyzed organic transformations, four‐coordinated planar cobalt complexes represent an active area of research due to their low cost, abundance on earth, and unique catalytic activity based on the three oxidation states of cobalt. These complexes can behave as various reactive intermediates, such as Co(II) metalloradicals, Co(III) hydrides, Co(I) bases, and Co(I) nucleophiles, according to the ligand field theory. For instance, planar Co(II) species have a d7 electronic configuration and act as metalloradical catalysts, which react with diazo and azide compounds to form reactive intermediates such as Co(III) carbene‐radicals and Co(III) nitrene‐radicals, respectively. These intermediates enable efficient cyclopropanation and aziridination of alkenes and C(sp3)‐H bond functionalization. Furthermore, the hydrogen bonding interaction between amide groups of planar ligands and polar substituents of the carbon radical on the Co(III) carbene‐radicals not only creates robust chiral cavities but also contributes to the stabilization of the Co(III) carbene‐radical and the transition state, consequently resulting in a dramatic improvement in reaction efficiency. The Co(III)‐hydrides provide a useful method for the generation of carbon radicals through hydrocobaltation of alkenes followed by homolytic cleavage of the Co−C bond. The carbon radical formation can participate in various hydrofunctionalizations and alkene‐isomerization. The d8 planar Co(I) complexes act as a base because they have an electronically filled dz2 orbital as the highest occupied molecular orbital (HOMO). This basicity enables various aromatic C−H functionalizations without a stoichiometric amount of oxidant. In contrast, the same planar Co(I) also acts as a superior nucleophilic catalyst, which can react with carbon electrophiles such as epoxides and organic (pseudo)halides to produce the corresponding alkyl‐Co(III) species. The generated alkyl‐Co(III) can be converted into the carbon radical or undergo transalkylation reaction with other transition metal catalysts. The strategies provide a new method for organic transformation with carbon electrophiles. This minireview covers recent developments in the field of four‐coordinated planar cobalt‐catalyzed organic transformations, paying particular attention to the relationship between their catalytic activities and oxidation states. The reactions have been categorized into those involving planar Co(II) complexes, Co(III) hydrides, and Co(I) complexes, with representative examples and insightful mechanistic discussions.
中文翻译:

有机合成中四配位平面钴催化的最新研究进展
在过渡金属催化的有机转化领域,由于四价平面钴配合物的低成本,地球上的丰富度以及基于钴的三种氧化态的独特催化活性,它们是研究的活跃领域。根据配体场论,这些络合物可以充当各种反应性中间体,例如Co(II)金属铁,Co(III)氢化物,Co(I)碱和Co(I)亲核试剂。例如,平面Co(II)物种具有ad 7电子构型并充当金属铁催化剂,它们与重氮和叠氮化物化合物反应形成反应性中间体,例如分别为Co(III)卡宾-基自由基和Co(III)氮-基自由基。 。这些中间体可实现烯烃和C(sp 3的高效环丙烷化和叠氮化)-H键功能化。此外,Co(III)卡宾基自由基上平面配体的酰胺基团与碳自由基的极性取代基之间的氢键相互作用不仅会产生坚固的手性空腔,而且还有助于Co(III)卡宾基自由基和碳原子的稳定。过渡态,因此导致反应效率显着提高。Co(III)-氢化物提供了一种有用的方法,可通过烯烃的加氢钴化反应,然后Co-C键的均相裂解来生成碳自由基。碳自由基的形成可以参与各种加氢官能化和烯烃异构化。d 8平面Co(I)配合物充当碱,因为它们具有电子填充的d z2轨道为最高占据分子轨道(HOMO)。这种碱性可以实现各种芳族CH官能化,而无需化学计量的氧化剂。相比之下,相同的平面Co(I)也是一种优良的亲核催化剂,可以与碳亲电试剂(例如环氧化物和有机(伪)卤化物)反应生成相应的烷基-Co(III)物种。生成的烷基-Co(III)可以转化为碳自由基或与其他过渡金属催化剂进行烷基转移反应。该策略为用碳亲电试剂进行有机转化提供了一种新方法。这篇小型综述涵盖了四配位平面钴催化的有机转化领域的最新进展,特别关注了其催化活性与氧化态之间的关系。
更新日期:2019-12-04
中文翻译:

有机合成中四配位平面钴催化的最新研究进展
在过渡金属催化的有机转化领域,由于四价平面钴配合物的低成本,地球上的丰富度以及基于钴的三种氧化态的独特催化活性,它们是研究的活跃领域。根据配体场论,这些络合物可以充当各种反应性中间体,例如Co(II)金属铁,Co(III)氢化物,Co(I)碱和Co(I)亲核试剂。例如,平面Co(II)物种具有ad 7电子构型并充当金属铁催化剂,它们与重氮和叠氮化物化合物反应形成反应性中间体,例如分别为Co(III)卡宾-基自由基和Co(III)氮-基自由基。 。这些中间体可实现烯烃和C(sp 3的高效环丙烷化和叠氮化)-H键功能化。此外,Co(III)卡宾基自由基上平面配体的酰胺基团与碳自由基的极性取代基之间的氢键相互作用不仅会产生坚固的手性空腔,而且还有助于Co(III)卡宾基自由基和碳原子的稳定。过渡态,因此导致反应效率显着提高。Co(III)-氢化物提供了一种有用的方法,可通过烯烃的加氢钴化反应,然后Co-C键的均相裂解来生成碳自由基。碳自由基的形成可以参与各种加氢官能化和烯烃异构化。d 8平面Co(I)配合物充当碱,因为它们具有电子填充的d z2轨道为最高占据分子轨道(HOMO)。这种碱性可以实现各种芳族CH官能化,而无需化学计量的氧化剂。相比之下,相同的平面Co(I)也是一种优良的亲核催化剂,可以与碳亲电试剂(例如环氧化物和有机(伪)卤化物)反应生成相应的烷基-Co(III)物种。生成的烷基-Co(III)可以转化为碳自由基或与其他过渡金属催化剂进行烷基转移反应。该策略为用碳亲电试剂进行有机转化提供了一种新方法。这篇小型综述涵盖了四配位平面钴催化的有机转化领域的最新进展,特别关注了其催化活性与氧化态之间的关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号