当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Equilibrium solubility, solvent effect and preferential solvation of 5-nitrofurazone (form γ) in aqueous co-solvent mixtures of isopropanol, N-methyl pyrrolidone, ethanol and dimethyl sulfoxide
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jct.2019.106016 Yuxin Bao , Ali Farajtabar , Min Zheng , Hongkun Zhao
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jct.2019.106016 Yuxin Bao , Ali Farajtabar , Min Zheng , Hongkun Zhao
|
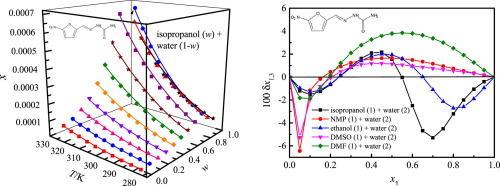
Abstract Solubilities of 5-nitrofurazone (form γ) in aqueous co-solvents mixtures of isopropanol, N-methyl pyrrolidone (NMP), ethanol and dimethyl sulfoxide (DMSO) were experimentally investigated via the saturation shake-flask technique at temperatures from 278.15 K to 323.15 K under ambient pressure of p = 101.2 kPa. The solubility of 5-nitrofurazone increased to a maximum value with 100% mass fractions of NMP and DMSO in aqueous solutions of NMP and DMSO, while with 80% mass fractions of isopropanol and ethanol in aqueous solutions of isopropanol and ethanol. The solids equilibrated with liquor were characterized by X-ray power diffraction, indicating that no solvate formation or polymorphic transformation occurred. The Jouyban-Acree model was accepted to correlate the obtained solubility. The acquired highest relative average deviation and root-mean-square deviation values were, respectively, 5.62 × 10−2 and 11.18 × 10−4. Quantitative values for local mole fractions of NMP (ethanol, DMSO, DMF or isopropanol) and water nearby 5-nitrofurazone were obtained by the method of Inverse Kirkwood–Buff integrals. The parameters of preferential solvation were positive for isopropanol/ethanol in the isopropanol/ethanol mixtures in intermediate compositions, and for NMP/DMF/DMSO in the NMP/DMF/DMSO mixtures in intermediate and NMP/DMF/DMSO-rich compositions, which indicated that 5-nitrofurazone was preferentially solvated by the co-solvents. Linear solvation energy relationships were used to model the solvent effect through the analysis of correlations between solubility data and solvent polarity quantified by suitable descriptors.
中文翻译:

5-硝基呋喃酮(γ型)在异丙醇、N-甲基吡咯烷酮、乙醇和二甲亚砜的水性共溶剂混合物中的平衡溶解度、溶剂效应和优先溶剂化
摘要 通过饱和摇瓶技术,在 278.15 K 至 278.15 K 的温度下实验研究了 5-硝基呋喃酮(γ 型)在异丙醇、N-甲基吡咯烷酮 (NMP)、乙醇和二甲亚砜 (DMSO) 的水性共溶剂混合物中的溶解度。在 p = 101.2 kPa 的环境压力下为 323.15 K。当NMP和DMSO的质量分数为100%时,5-硝基呋喃酮在NMP和DMSO水溶液中的溶解度达到最大值,而异丙醇和乙醇的质量分数为80%时,在异丙醇和乙醇的水溶液中。用液体平衡的固体通过 X 射线功率衍射表征,表明没有溶剂化物形成或多晶型转变发生。Jouyban-Acree 模型被接受来关联获得的溶解度。获得的最高相对平均偏差和均方根偏差值分别为 5.62 × 10-2 和 11.18 × 10-4。NMP(乙醇、DMSO、DMF 或异丙醇)和 5-硝基呋喃酮附近的水的局部摩尔分数的定量值是通过逆柯克伍德-布夫积分的方法获得的。中间组合物中异丙醇/乙醇混合物中的异丙醇/乙醇的优先溶剂化参数为正,中间组合物中的 NMP/DMF/DMSO 混合物中的 NMP/DMF/DMSO 和富含 NMP/DMF/DMSO 的组合物的优先溶剂化参数为正值,这表明5-硝基呋喃酮优先被共溶剂溶剂化。通过分析溶解度数据和由合适描述符量化的溶剂极性之间的相关性,使用线性溶剂化能关系来模拟溶剂效应。
更新日期:2020-01-01
中文翻译:

5-硝基呋喃酮(γ型)在异丙醇、N-甲基吡咯烷酮、乙醇和二甲亚砜的水性共溶剂混合物中的平衡溶解度、溶剂效应和优先溶剂化
摘要 通过饱和摇瓶技术,在 278.15 K 至 278.15 K 的温度下实验研究了 5-硝基呋喃酮(γ 型)在异丙醇、N-甲基吡咯烷酮 (NMP)、乙醇和二甲亚砜 (DMSO) 的水性共溶剂混合物中的溶解度。在 p = 101.2 kPa 的环境压力下为 323.15 K。当NMP和DMSO的质量分数为100%时,5-硝基呋喃酮在NMP和DMSO水溶液中的溶解度达到最大值,而异丙醇和乙醇的质量分数为80%时,在异丙醇和乙醇的水溶液中。用液体平衡的固体通过 X 射线功率衍射表征,表明没有溶剂化物形成或多晶型转变发生。Jouyban-Acree 模型被接受来关联获得的溶解度。获得的最高相对平均偏差和均方根偏差值分别为 5.62 × 10-2 和 11.18 × 10-4。NMP(乙醇、DMSO、DMF 或异丙醇)和 5-硝基呋喃酮附近的水的局部摩尔分数的定量值是通过逆柯克伍德-布夫积分的方法获得的。中间组合物中异丙醇/乙醇混合物中的异丙醇/乙醇的优先溶剂化参数为正,中间组合物中的 NMP/DMF/DMSO 混合物中的 NMP/DMF/DMSO 和富含 NMP/DMF/DMSO 的组合物的优先溶剂化参数为正值,这表明5-硝基呋喃酮优先被共溶剂溶剂化。通过分析溶解度数据和由合适描述符量化的溶剂极性之间的相关性,使用线性溶剂化能关系来模拟溶剂效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号