当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective chemoenzymatic synthesis of (R)-γ-valerolactone from levulinic acid
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.procbio.2019.11.019 Dohoon Lee , Young Joo Yeon
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.procbio.2019.11.019 Dohoon Lee , Young Joo Yeon
|
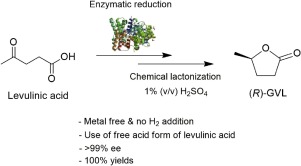
Abstract A number of chemicals with high industrial value can be synthesized from levulinic acid, a feasible building block readily available from cellulosic biomass. Among them, γ-valerolactone is a versatile chemical precursor for the synthesis of value-added products including bio-active molecules, bio-fuels, and carbon-based chemicals. In this study, a novel two-step chemoenzymatic conversion of levulinic acid to (R)-γ-valerolactone via 4-hydroxyvaleric acid was investigated. For that purpose, an engineered 3-hydroxybutyrate dehydrogenase (e3HBDH) with improved catalytic activity toward levulinic acid was employed in the first-step reaction, and dehydration with 1 % (v/v) sulfuric acid was applied for the lactonization of 4-hydroxyvaleric acid to γ-valerolactone in the second step. As a result, enantiomerically pure (R)-γ-valerolactone (>99 % ee) was successfully produced from the free acid form of levulinic acid with the maximum yield of approximately 100 %.
中文翻译:

从乙酰丙酸对映选择性化学酶法合成 (R)-γ-戊内酯
摘要 许多具有高工业价值的化学品可以从乙酰丙酸合成,乙酰丙酸是一种易于从纤维素生物质中获得的可行构件。其中,γ-戊内酯是一种通用的化学前体,用于合成高附加值产品,包括生物活性分子、生物燃料和碳基化学品。在这项研究中,研究了一种通过 4-羟基戊酸将乙酰丙酸转化为 (R)-γ-戊内酯的新型两步化学酶促转化。为此,在第一步反应中采用了对乙酰丙酸具有更高催化活性的工程化 3-羟基丁酸脱氢酶 (e3HBDH),并使用 1% (v/v) 硫酸脱水用于 4-羟基戊酸的内酯化第二步酸化为γ-戊内酯。因此,对映体纯 (R)-γ-戊内酯 (>
更新日期:2020-03-01
中文翻译:

从乙酰丙酸对映选择性化学酶法合成 (R)-γ-戊内酯
摘要 许多具有高工业价值的化学品可以从乙酰丙酸合成,乙酰丙酸是一种易于从纤维素生物质中获得的可行构件。其中,γ-戊内酯是一种通用的化学前体,用于合成高附加值产品,包括生物活性分子、生物燃料和碳基化学品。在这项研究中,研究了一种通过 4-羟基戊酸将乙酰丙酸转化为 (R)-γ-戊内酯的新型两步化学酶促转化。为此,在第一步反应中采用了对乙酰丙酸具有更高催化活性的工程化 3-羟基丁酸脱氢酶 (e3HBDH),并使用 1% (v/v) 硫酸脱水用于 4-羟基戊酸的内酯化第二步酸化为γ-戊内酯。因此,对映体纯 (R)-γ-戊内酯 (>









































 京公网安备 11010802027423号
京公网安备 11010802027423号