当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron Paramagnetic Resonance Spectroscopic Identification of the Fe-S Clusters in the SPASM Domain-Containing Radical SAM Enzyme PqqE.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-11 , DOI: 10.1021/acs.biochem.9b00960 Lizhi Tao 1 , Wen Zhu 2 , Judith P Klinman 2 , R David Britt 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-11 , DOI: 10.1021/acs.biochem.9b00960 Lizhi Tao 1 , Wen Zhu 2 , Judith P Klinman 2 , R David Britt 1
Affiliation
|
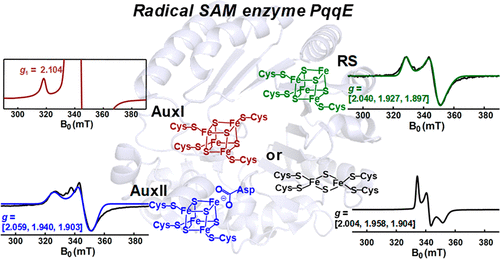
Pyrroloquinoline quinone (PQQ) is an important redox active quinocofactor produced by a wide variety of bacteria. A key step in PQQ biosynthesis is a carbon-carbon cross-link reaction between glutamate and tyrosine side chains within the ribosomally synthesized peptide substrate PqqA. This reaction is catalyzed by the radical SAM enzyme PqqE. Previous X-ray crystallographic and spectroscopic studies suggested that PqqE, like the other members of the SPASM domain family, contains two auxiliary Fe-S clusters (AuxI and AuxII) in addition to the radical SAM [4Fe-4S] cluster. However, a clear assignment of the electron paramagnetic resonance (EPR) signal of each Fe-S cluster was hindered by the isolation of a His6-tagged PqqE variant with an altered AuxI cluster. In this work, we are able to isolate soluble PqqE variants by using a less disruptive strep-tactin chromatographic approach. We have unambiguously identified the EPR signatures for four forms of Fe-S clusters present in PqqE through the use of multifrequency EPR spectroscopy: the RS [4Fe-4S] cluster, the AuxII [4Fe-4S] cluster, and two different clusters ([4Fe-4S] and [2Fe-2S]) bound in the AuxI site. The RS [4Fe-4S] cluster, the AuxII [4Fe-4S] cluster, and the [2Fe-2S] cluster form in the AuxI site can all be reduced by sodium dithionite, with g tensors of their reduced form determined as [2.040, 1.927, 1.897], [2.059, 1.940, 1.903], and [2.004, 1.958, 1.904], respectively. The AuxI [4Fe-4S] cluster that is determined on the basis of its relaxation profile can be reduced only by using low-potential reductants such as Ti(III) citrate or Eu(II)-DTPA to give rise to a g1 = 2.104 signal. Identification of the EPR signature for each cluster paves the way for further investigations of SPASM domain radical SAM enzymes.
中文翻译:

电子顺磁共振波谱识别包含SPASM域的自由基SAM酶PqqE中的Fe-S团簇。
吡咯并喹啉醌(PQQ)是由多种细菌产生的重要的氧化还原活性喹啉。PQQ生物合成的关键步骤是核糖体合成的肽底物PqqA中谷氨酸和酪氨酸侧链之间的碳-碳交联反应。该反应由自由基SAM酶PqqE催化。先前的X射线晶体学和光谱学研究表明,与SPASM域家族的其他成员一样,PqqE除了基团SAM [4Fe-4S]团簇外,还包含两个辅助Fe-S簇(AuxI和AuxII)。然而,通过分离带有改变的AuxI簇的His6标记的PqqE变体,阻碍了每个Fe-S簇的电子顺磁共振(EPR)信号的明确分配。在这项工作中,我们能够通过使用破坏性较小的链球菌-肌动蛋白色谱法分离可溶性PqqE变体。我们已经通过使用多频EPR光谱法明确地确定了PqqE中存在的四种形式的Fe-S团簇的EPR签名:RS [4Fe-4S]团簇,AuxII [4Fe-4S]团簇和两个不同的团簇([ (4Fe-4S]和[2Fe-2S])绑定在AuxI位点中。连二亚硫酸钠可还原AuxI位点中的RS [4Fe-4S]团簇,AuxII [4Fe-4S]团簇和[2Fe-2S]团簇形式,其还原形式的g张量确定为[2.040] ,1.927、1.897,[2.059、1.940、1.903]和[2.004、1.958、1.904]。仅通过使用低电位还原剂(例如柠檬酸Ti(III)或Eu(II)-DTPA)产生基于auxI [4Fe-4S]簇的弛豫分布,可以降低g1 = 2.104信号。鉴定每个簇的EPR标记为进一步研究SPASM域自由基SAM酶铺平了道路。
更新日期:2019-12-11
中文翻译:

电子顺磁共振波谱识别包含SPASM域的自由基SAM酶PqqE中的Fe-S团簇。
吡咯并喹啉醌(PQQ)是由多种细菌产生的重要的氧化还原活性喹啉。PQQ生物合成的关键步骤是核糖体合成的肽底物PqqA中谷氨酸和酪氨酸侧链之间的碳-碳交联反应。该反应由自由基SAM酶PqqE催化。先前的X射线晶体学和光谱学研究表明,与SPASM域家族的其他成员一样,PqqE除了基团SAM [4Fe-4S]团簇外,还包含两个辅助Fe-S簇(AuxI和AuxII)。然而,通过分离带有改变的AuxI簇的His6标记的PqqE变体,阻碍了每个Fe-S簇的电子顺磁共振(EPR)信号的明确分配。在这项工作中,我们能够通过使用破坏性较小的链球菌-肌动蛋白色谱法分离可溶性PqqE变体。我们已经通过使用多频EPR光谱法明确地确定了PqqE中存在的四种形式的Fe-S团簇的EPR签名:RS [4Fe-4S]团簇,AuxII [4Fe-4S]团簇和两个不同的团簇([ (4Fe-4S]和[2Fe-2S])绑定在AuxI位点中。连二亚硫酸钠可还原AuxI位点中的RS [4Fe-4S]团簇,AuxII [4Fe-4S]团簇和[2Fe-2S]团簇形式,其还原形式的g张量确定为[2.040] ,1.927、1.897,[2.059、1.940、1.903]和[2.004、1.958、1.904]。仅通过使用低电位还原剂(例如柠檬酸Ti(III)或Eu(II)-DTPA)产生基于auxI [4Fe-4S]簇的弛豫分布,可以降低g1 = 2.104信号。鉴定每个簇的EPR标记为进一步研究SPASM域自由基SAM酶铺平了道路。









































 京公网安备 11010802027423号
京公网安备 11010802027423号