当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of hydrazinodihydrofurans via cascade Michael addition-substitution involving the reaction of curcumin and other β-dicarbonyls with α-hydrazinonitroalkenes.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-11-26 , DOI: 10.1039/c9ob01974j Kalisankar Bera 1 , Narasimham Ayyagari , Nishikant Satam , Irishi N N Namboothiri
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-11-26 , DOI: 10.1039/c9ob01974j Kalisankar Bera 1 , Narasimham Ayyagari , Nishikant Satam , Irishi N N Namboothiri
Affiliation
|
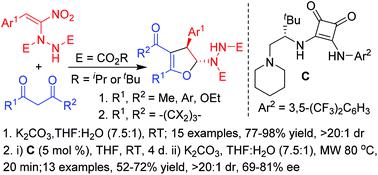
Highly diastereoselective synthesis of 2-hydrazinated 2,3-dihydrofurans in good to excellent yields involving an interrupted Feist–Bénary type reaction by treating a wide variety of 1,3-dicarbonyl compounds, including curcumins, with α-hydrazinated nitroalkenes is reported here. The first ever enantioselective reaction of α-hydrazinonitroalkenes has also been carried out with two selected 1,3-dicarbonyls, dimedone and cyclohexanone by employing an L-t-leucine derived squaramide as the chiral organocatalyst to afford the enantio-enriched 2-hydrazinodihydrofurans as single diastereomers in good yields and with good enantioselectivities.
中文翻译:

经由级联迈克尔加成取代的立体选择性合成肼二氢呋喃,涉及姜黄素和其他β-二羰基与α-肼基硝基烯烃的反应。
据报道,通过α-肼化硝基烯烃处理各种1,3-二羰基化合物(包括姜黄素),可以中断Feist-Bénary型反应,以高至极好的产率对2-肼化2,3-二氢呋喃进行高度非对映选择性合成。α-hydrazinonitroalkenes有史以来的第一次对映体选择性反应也已通过采用具有两个选择的1,3-二羰基,双甲酮和环己酮进行大号-吨-亮氨酸squaramide衍生作为手性有机催化剂,得到对映富集的2- hydrazinodihydrofurans作为单一的非对映异构体,具有良好的收率和良好的对映选择性。
更新日期:2019-11-26
中文翻译:

经由级联迈克尔加成取代的立体选择性合成肼二氢呋喃,涉及姜黄素和其他β-二羰基与α-肼基硝基烯烃的反应。
据报道,通过α-肼化硝基烯烃处理各种1,3-二羰基化合物(包括姜黄素),可以中断Feist-Bénary型反应,以高至极好的产率对2-肼化2,3-二氢呋喃进行高度非对映选择性合成。α-hydrazinonitroalkenes有史以来的第一次对映体选择性反应也已通过采用具有两个选择的1,3-二羰基,双甲酮和环己酮进行大号-吨-亮氨酸squaramide衍生作为手性有机催化剂,得到对映富集的2- hydrazinodihydrofurans作为单一的非对映异构体,具有良好的收率和良好的对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号