Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.38137 Bo Wang 1, 2 , Yingke Zhou 1, 2 , Jun Zhang 3 , Xin Jin 2, 4 , Heshui Wu 1 , Haojie Huang 2, 5, 6
|
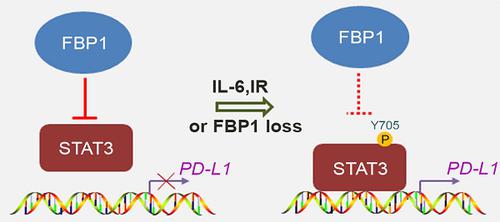
Rationale: Abnormal expression of programmed death-1 (PD-1) ligand-1(PD-L1) in cancer cells plays a crucial role in cancer immune evasion and progression. The immune checkpoint molecules PD-1 and PD-L1 have been targeted for cancer treatment with significant benefits for cancer patients. However, the response rate is relatively low in certain types of cancer and the underlying mechanism remains poorly understood. Better understanding of the molecular mechanism of PD-L1 expression regulation in cancer cells is urgently needed to improve the treatment response rate and overall survival of patients. Fructose-1, 6-biphosphatase (FBP1) is a key enzyme in gluconeogenesis and is implicated in human cancer due to its frequent loss in various cancer types.
Methods: Expression of FBP1 and PD-L1 was analyzed in various cancer cell lines. Western blot and RT-qPCR were performed to determine whether FBP1 regulates PD-L1 expression. Co-immunoprecipitation and glutathione S-transferase (GST) pulldown assay were employed to define the underlying regulatory mechanisms. Immunohistochemistry was conducted to determine the correlation between FBP1 and PD-L1 expression in a cohort of patients. A cancer syngeneic mouse model was utilized to examine how FBP1 affects tumor immunity.
Results: We demonstrated that in a manner independent of its enzymatic activity FBP1 downregulates the expression of PD-L1 in various cell lines of different cancer types including pancreatic and prostate cancer. We further showed that this regulation occurs at the transcriptional level and is mediated by FBP1 inhibition of signal transducer and activator of transcription-3 (STAT3)-dependent PD-L1 transcription. Moreover, FBP1 and PD-L1 protein expression were negatively correlated in pancreatic ductal adenocarcinoma (PDAC) specimens from a cohort of patients. Most importantly, we demonstrated that decreased FBP1 expression promotes tumor growth and resistance to immune checkpoint blockade therapy in mice.
Conclusions: Our findings reveal a new tumor suppressor function of FBP1 in inhibiting PD-L1 expression and enhancing cancer immunity. They also suggest that FBP1-deficient human cancers could be therapeutically targeted by PD-1/PD-L1-based immune checkpoint blockade therapy.
中文翻译:

果糖1,6-双磷酸酶的丢失调节了STAT3依赖的PD-L1表达和癌症免疫力。
理由:癌细胞中程序性死亡1(PD-1)配体1(PD-L1)的异常表达在癌症免疫逃避和进展中起着至关重要的作用。免疫检查点分子PD-1和PD-L1已被靶向用于癌症治疗,对癌症患者具有明显的益处。但是,在某些类型的癌症中,缓解率相对较低,其潜在机制仍知之甚少。迫切需要更好地了解癌细胞中PD-L1表达调节的分子机制,以提高患者的治疗反应率和总体生存率。1、6-二磷酸果糖酶(FBP1)是糖异生中的关键酶,由于其在各种癌症类型中的频繁丢失而与人类癌症有关。
方法:分析FBP1和PD-L1在各种癌细胞系中的表达。进行蛋白质印迹和RT-qPCR以确定FBP1是否调节PD-L1表达。共免疫沉淀和谷胱甘肽S-转移酶(GST)下拉测定法被用来定义潜在的调节机制。进行了免疫组织化学以确定一组患者中FBP1和PD-L1表达之间的相关性。利用癌症同系小鼠模型检查FBP1如何影响肿瘤免疫力。
结果:我们证明,FBP1以不依赖其酶促活性的方式下调PD-L1在不同癌症类型(包括胰腺癌和前列腺癌)的各种细胞系中的表达。我们进一步表明,这种调节发生在转录水平,并由FBP1抑制信号转导子和转录激活子(STAT3)依赖的PD-L1转录介导。此外,在一群患者的胰腺导管腺癌(PDAC)标本中,FBP1和PD-L1蛋白表达呈负相关。最重要的是,我们证明了降低的FBP1表达促进了小鼠的肿瘤生长和对免疫检查点封锁疗法的抵抗力。
结论:我们的发现揭示了FBP1在抑制PD-L1表达和增强癌症免疫力方面具有新的抑癌功能。他们还暗示,基于PD-1 / PD-L1的免疫检查点封锁疗法可以靶向治疗FBP1缺乏的人类癌症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号