Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.40076 Xueran Chen 1, 2 , Aijun Hao 3 , Xian Li 4 , Kaiqin Ye 1, 2 , Chenggang Zhao 1, 5 , Haoran Yang 1, 5 , Huihui Ma 6 , Lei Hu 1, 5 , Zhiyang Zhao 1, 5 , Lizhu Hu 1, 5 , Fang Ye 2 , Qiuyan Sun 2 , Huaman Zhang 2 , Hongzhi Wang 1, 2 , Xuebiao Yao 7 , Zhiyou Fang 1, 2
|
Rationale: Glioblastoma multiforme (GBM) almost invariably gain invasive phenotype with limited therapeutic strategy and ill-defined mechanism. By studying the aberrant expression landscape of gliomas, we find significant up-regulation of p-MAPK level in GBM and a potent independent prognostic marker for overall survival. DHHC family was generally expressed in glioma and closely related to the activation of MAPK signaling pathway, but its role and clinical significance in GBM development and malignant progression are yet to be determined.
Method: Bioinformatics analysis, western blotting and immunohistochemistry (IHC) were performed to detect the expression of ZDHHC17 in GBM. The biological function of ZDHHC17 was demonstrated by a series of in vitro and in vivo experiments. Pharmacological treatment, flow cytometry, Transwell migration assay, Co- Immunoprecipitation and GST pulldown were carried out to demonstrate the potential mechanisms of ZDHHC17.
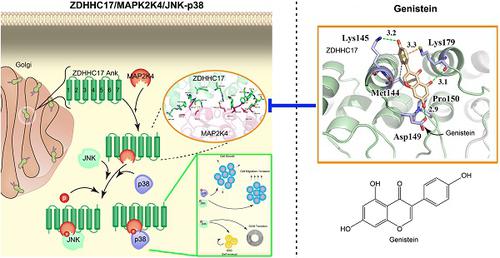
Results: ZDHHC17 is up-regulated and coordinated with MAPK activation in GBM. Mechanistically, ZDHHC17 interacts with MAP2K4 and p38/JNK to build a signaling module for MAPK activation and malignant progression. Notably, the ZDHHC17-MAP2K4-JNK/p38 signaling module contributes to GBM development and malignant progression by promoting GBM cell tumorigenicity and glioma stem cell (GSC) self-renewal. Moreover, we identify a small molecule, genistein, as a specific inhibitor to disrupt ZDHHC17-MAP2K4 complex formation for GBM cell proliferation and GSC self-renewal. Moreover, genistein, identified herein as a lead candidate for ZDHHC17-MAP2K4 inhibition, demonstrated potential therapeutic effect in patients with ZDHHC17-expressing GBM.
Conclusions: Our study identified disruption of a previously unrecognized signaling module as a target strategy for GBM treatment, and provided direct evidence of the efficacy of its inhibition in glioma using a specific inhibitor.
中文翻译:

ZDHHC17介导的JNK和p38 MAPK的激活驱动胶质母细胞瘤多形发育和恶性进展。
理由:多形性胶质母细胞瘤(GBM)几乎总是获得侵袭性表型,其治疗策略和机制尚不明确。通过研究神经胶质瘤的异常表达情况,我们发现GBM中p -MAPK水平显着上调,并且是整体生存的有效独立预后标志物。DHHC家族通常在神经胶质瘤中表达,与MAPK信号通路的激活密切相关,但是其在GBM形成和恶性进展中的作用和临床意义尚待确定。
方法:采用生物信息学分析,免疫印迹和免疫组化(IHC)法检测ZDHHC17在GBM中的表达。通过一系列的体外和体内实验证明了ZDHHC17的生物学功能。进行了药理学处理,流式细胞仪,Transwell迁移测定,共免疫沉淀和GST下拉实验,以证明ZDHHC17的潜在机制。
结果: ZDHHC17上调并与GBM中的MAPK激活相协调。从机制上讲,ZDHHC17与MAP2K4和p38 / JNK相互作用以构建用于MAPK激活和恶性进展的信号传导模块。值得注意的是,ZDHHC17-MAP2K4-JNK / p38信号传导模块通过促进GBM细胞致瘤性和神经胶质瘤干细胞(GSC)自我更新,促进了GBM的发展和恶性进展。此外,我们确定了一个小分子染料木黄酮,作为破坏GBM细胞增殖和GSC自我更新的ZDHHC17-MAP2K4复合物形成的特异性抑制剂。此外,染料木黄酮(在本文中被确定为ZDHHC17-MAP2K4抑制的主要候选药物)在表达ZDHHC17的GBM患者中显示出潜在的治疗作用。
结论:我们的研究确定破坏先前无法识别的信号传导模块是GBM治疗的目标策略,并提供了使用特定抑制剂抑制其对神经胶质瘤疗效的直接证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号