Oncogenesis ( IF 5.9 ) Pub Date : 2019-11-26 , DOI: 10.1038/s41389-019-0178-3 Hironobu Yamashita 1 , Yuka I Kawasawa 2, 3, 4 , Lauren Shuman 1, 5 , Zongyu Zheng 1 , Truc Tran 1 , Vonn Walter 6 , Joshua I Warrick 1, 5 , Guoli Chen 1 , Hikmat Al-Ahmadie 7 , Matthew Kaag 5 , Pak Kin Wong 8 , Jay D Raman 5 , David J DeGraff 1, 4, 5
|
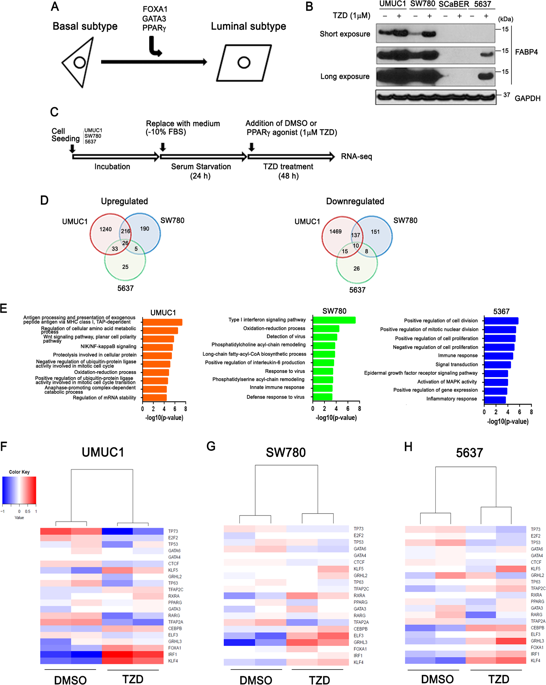
The discovery of bladder cancer transcriptional subtypes provides an opportunity to identify high risk patients, and tailor disease management. Recent studies suggest tumor heterogeneity contributes to regional differences in molecular subtype within the tumor, as well as during progression and following treatment. Nonetheless, the transcriptional drivers of the aggressive basal-squamous subtype remain unidentified. As PPARɣ has been repeatedly implicated in the luminal subtype of bladder cancer, we hypothesized inactivation of this transcriptional master regulator during progression results in increased expression of basal-squamous specific transcription factors (TFs) which act to drive aggressive behavior. We initiated a pharmacologic and RNA-seq-based screen to identify PPARɣ-repressed, basal-squamous specific TFs. Hierarchical clustering of RNA-seq data following treatment of three human bladder cancer cells with a PPARɣ agonist identified a number of TFs regulated by PPARɣ activation, several of which are implicated in urothelial and squamous differentiation. One PPARɣ-repressed TF implicated in squamous differentiation identified is Transcription Factor Activating Protein 2 alpha (TFAP2A). We show TFAP2A and its paralog TFAP2C are overexpressed in basal-squamous bladder cancer and in squamous areas of cystectomy samples, and that overexpression is associated with increased lymph node metastasis and distant recurrence, respectively. Biochemical analysis confirmed the ability of PPARɣ activation to repress TFAP2A, while PPARɣ antagonist and PPARɣ siRNA knockdown studies indicate the requirement of a functional receptor. In vivo tissue recombination studies show TFAP2A and TFAP2C promote tumor growth in line with the aggressive nature of basal-squamous bladder cancer. Our findings suggest PPARɣ inactivation, as well as TFAP2A and TFAP2C overexpression cooperate with other TFs to promote the basal-squamous transition during tumor progression.
中文翻译:

PPARγ抑制转录因子AP-2α揭示了基底鳞癌的新型转录回路。
膀胱癌转录亚型的发现为识别高危患者和调整疾病管理提供了机会。最近的研究表明,肿瘤异质性导致了肿瘤内以及进展过程和后续治疗中分子亚型的区域差异。尽管如此,侵袭性基底鳞状亚型的转录驱动因子仍未确定。由于PPARɣ已经反复涉及膀胱癌的管腔亚型,我们推测该转录主调节因子的失活在进展过程中会导致基底鳞状特异性转录因子(TFs)表达的增加,而这些转录因子的作用是驱动侵略性行为。我们启动了基于药理学和基于RNA seq的筛选,以鉴定PPARɣ抑制的基底鳞状特异性TF。用PPARα激动剂处理三个人膀胱癌细胞后,RNA-seq数据的分层聚类确定了许多受PPARβ激活调节的TF,其中一些与尿路上皮和鳞状细胞分化有关。鉴定出的一种与鳞状细胞分化有关的PPAR 3抑制的TF是转录因子激活蛋白2α(TFAP2A)。我们显示TFAP2A及其旁系TFAP2C在基底鳞癌和膀胱切除术样本的鳞状区域中过表达,并且过表达分别与增加的淋巴结转移和远处复发有关。生化分析证实了PPARɣ激活具有抑制TFAP2A的能力,而PPARɣ拮抗剂和PPARɣsiRNA敲低研究表明需要功能性受体。体内组织重组研究显示,TFAP2A和TFAP2C促进肿瘤的生长符合基底鳞癌的侵袭性。我们的发现表明,PPARα失活以及TFAP2A和TFAP2C的过表达与其他TF协同作用,以促进肿瘤进展过程中的基底鳞状过渡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号