当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic insight into the rhodium(III)‐catalyzed ortho‐selective coupling of diverse arenes with 4‐acyl‐1‐sulfonyltriazoles: A computational study
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-26 , DOI: 10.1002/qua.26119 Weirong Wu 1, 2 , Yue Liu 1 , Jiayi Hu 1 , Jiageng Xiong 1 , Hua Hou 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-26 , DOI: 10.1002/qua.26119 Weirong Wu 1, 2 , Yue Liu 1 , Jiayi Hu 1 , Jiageng Xiong 1 , Hua Hou 1
Affiliation
|
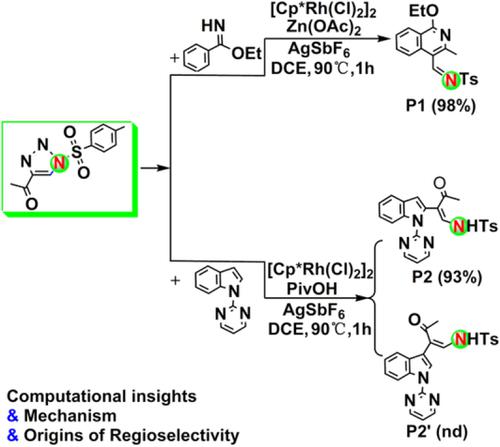
A mechanistic study of the Cp*Rh(III)‐catalyzed annulative coupling of benzimidates with 4‐acyl‐1‐sulfonyltriazoles by CH activation was performed using density functional M06 method. It was demonstrated that the active catalyst during the coupling process should be the cation [Cp*Rh(OAc)]+ rather than the neutral Cp*Rh(OAc)2 and Zn(OAc)2 as proposed previously by the experimenters. A novel energetically feasible reaction pathway has been revealed theoretically in details. The acetic acid‐mediated cyclization process was confirmed to be the rate‐limiting step with an overall barrier of 24.3 kcal/mol, excluding any importance of the CH cleavage mechanism as supported by the kinetic isotope effect experiments. The major factors responsible for the preferred regioselectivity of N‐pyrimidinylinoles with 4‐acetyl‐1‐sulfonyltriazoles were discussed.
中文翻译:

机械洞察铑(III)催化不同芳烃与4-酰基-1-磺酰基三唑的邻位选择性偶联:计算研究
使用密度泛函M06法对Cp * Rh(III)催化苯甲二酸酯与4-酰基-1-磺酰基三唑的CH活化的环状偶联进行了机理研究。结果表明,偶联过程中的活性催化剂应为阳离子[Cp * Rh(OAc)] +,而不是实验人员先前提出的中性Cp * Rh(OAc)2和Zn(OAc)2。理论上已经详细揭示了一种新的在能量上可行的反应途径。乙酸介导的环化方法被证实是限速步骤24.3千卡/摩尔的整体屏障,不包括作为C任何重要性H裂解机理受到动力学同位素效应实验的支持。讨论了影响N-嘧啶与4-乙酰基-1-磺酰基三唑的优选区域选择性的主要因素。
更新日期:2019-12-21
中文翻译:

机械洞察铑(III)催化不同芳烃与4-酰基-1-磺酰基三唑的邻位选择性偶联:计算研究
使用密度泛函M06法对Cp * Rh(III)催化苯甲二酸酯与4-酰基-1-磺酰基三唑的CH活化的环状偶联进行了机理研究。结果表明,偶联过程中的活性催化剂应为阳离子[Cp * Rh(OAc)] +,而不是实验人员先前提出的中性Cp * Rh(OAc)2和Zn(OAc)2。理论上已经详细揭示了一种新的在能量上可行的反应途径。乙酸介导的环化方法被证实是限速步骤24.3千卡/摩尔的整体屏障,不包括作为C任何重要性H裂解机理受到动力学同位素效应实验的支持。讨论了影响N-嘧啶与4-乙酰基-1-磺酰基三唑的优选区域选择性的主要因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号