当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heavy-Metals-Mediated Phospholipids Scrambling by Human Phospholipid Scramblase 3: A Probable Role in Mitochondrial Apoptosis.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-12-10 , DOI: 10.1021/acs.chemrestox.9b00406 Santosh Kumar Palanirajan 1 , Sathyanarayana N Gummadi 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-12-10 , DOI: 10.1021/acs.chemrestox.9b00406 Santosh Kumar Palanirajan 1 , Sathyanarayana N Gummadi 1
Affiliation
|
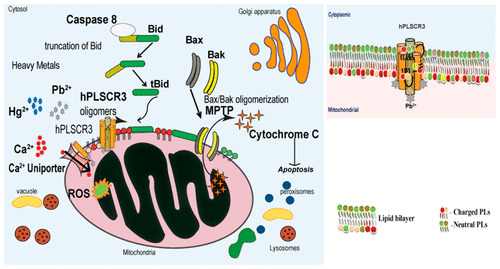
Human phospholipid scramblases are a family of four homologous transmembrane proteins (hPLSCR1-4) mediating phospholipids (PLs) translocation in plasma membrane upon Ca2+ activation. hPLSCR3, the only homologue localized to mitochondria, plays a vital role in mitochondrial structure, function, maintenance, and apoptosis. Upon Ca2+ activation, hPLSCR3 mediates PL translocation at the mitochondrial membrane enhancing t-bid-induced cytochrome c release and apoptosis. Mitochondria are important target organelles for heavy-metals-induced apoptotic signaling cascade and are the central executioner of apoptosis to trigger. Pb2+ and Hg2+ toxicity mediates apoptosis by increased reactive oxygen species (ROS) and cytochrome c release from mitochondria. To discover the role of hPLSCR3 in heavy metal toxicity, hPLSCR3 was overexpressed as a recombinant protein in Escherichia coli Rosetta (DE3) and purified by affinity chromatography. The biochemical assay using synthetic proteoliposomes demonstrated that hPLSCR3 translocated aminophospholipids in the presence of micromolar concentrations of Pb2+ and Hg2+. A point mutation in the Ca2+-binding motif (F258V) led to a ∼60% loss in the functional activity and decreased binding affinities for Pb2+ and Hg2+ implying that the divalent heavy metal ions bind to the Ca2+-binding motif. This was further affirmed by the characteristic spectra observed with stains-all dye. The conformational changes upon heavy metal binding were monitored by circular dichroism, intrinsic tryptophan fluorescence, and light-scattering studies. Our results revealed that Pb2+ and Hg2+ bind to hPLSCR3 with higher affinity than Ca2+ thus mediating scramblase activity. To summarize, this is the first biochemical evidence for heavy metals binding to the mitochondrial membrane protein leading to bidirectional translocation of PLs specifically toward phosphatidylethanolamine.
中文翻译:

重金属介导的磷脂被人的磷脂Scramblase扰乱3:可能在线粒体细胞凋亡中发挥作用。
人磷脂scramblases是四个同源跨膜蛋白(hPLSCR1-4)家族,介导Ca2 +活化后质膜中的磷脂(PLs)易位。hPLSCR3是唯一定位于线粒体的同源物,在线粒体的结构,功能,维持和凋亡中起着至关重要的作用。在Ca2 +激活后,hPLSCR3在线粒体膜上介导PL易位,从而增强t-bid诱导的细胞色素c释放和凋亡。线粒体是重金属诱导的凋亡信号级联反应的重要靶细胞器,并且是细胞凋亡触发的主要执行者。Pb2 +和Hg2 +毒性通过增加线粒体中的活性氧(ROS)和细胞色素c的释放来介导细胞凋亡。要发现hPLSCR3在重金属毒性中的作用,hPLSCR3作为重组蛋白在大肠杆菌Rosetta(DE3)中过表达,并通过亲和层析纯化。使用合成蛋白脂质体的生化分析表明,在微摩尔浓度的Pb2 +和Hg2 +的存在下,hPLSCR3易位的氨基磷脂。Ca2 +结合基序(F258V)中的点突变导致功能活性降低约60%,并且对Pb2 +和Hg2 +的结合亲和力降低,这表明二价重金属离子与Ca2 +结合基序结合。用所有污渍染料观察到的特征光谱进一步证实了这一点。重金属结合时的构象变化通过圆二色性,固有色氨酸荧光和光散射研究进行监测。我们的研究结果表明,Pb2 +和Hg2 +与hPLSCR3的亲和力高于Ca2 +,从而介导了scramblase活性。综上所述,这是重金属与线粒体膜蛋白结合的首个生化证据,可导致PL特异地向磷脂酰乙醇胺双向迁移。
更新日期:2019-12-11
中文翻译:

重金属介导的磷脂被人的磷脂Scramblase扰乱3:可能在线粒体细胞凋亡中发挥作用。
人磷脂scramblases是四个同源跨膜蛋白(hPLSCR1-4)家族,介导Ca2 +活化后质膜中的磷脂(PLs)易位。hPLSCR3是唯一定位于线粒体的同源物,在线粒体的结构,功能,维持和凋亡中起着至关重要的作用。在Ca2 +激活后,hPLSCR3在线粒体膜上介导PL易位,从而增强t-bid诱导的细胞色素c释放和凋亡。线粒体是重金属诱导的凋亡信号级联反应的重要靶细胞器,并且是细胞凋亡触发的主要执行者。Pb2 +和Hg2 +毒性通过增加线粒体中的活性氧(ROS)和细胞色素c的释放来介导细胞凋亡。要发现hPLSCR3在重金属毒性中的作用,hPLSCR3作为重组蛋白在大肠杆菌Rosetta(DE3)中过表达,并通过亲和层析纯化。使用合成蛋白脂质体的生化分析表明,在微摩尔浓度的Pb2 +和Hg2 +的存在下,hPLSCR3易位的氨基磷脂。Ca2 +结合基序(F258V)中的点突变导致功能活性降低约60%,并且对Pb2 +和Hg2 +的结合亲和力降低,这表明二价重金属离子与Ca2 +结合基序结合。用所有污渍染料观察到的特征光谱进一步证实了这一点。重金属结合时的构象变化通过圆二色性,固有色氨酸荧光和光散射研究进行监测。我们的研究结果表明,Pb2 +和Hg2 +与hPLSCR3的亲和力高于Ca2 +,从而介导了scramblase活性。综上所述,这是重金属与线粒体膜蛋白结合的首个生化证据,可导致PL特异地向磷脂酰乙醇胺双向迁移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号