当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiols in Natural Organic Matter: Molecular Forms, Acidity, and Reactivity with Mercury(II) from First-Principles Calculations and High Energy-Resolution X-ray Absorption Near-Edge Structure Spectroscopy
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-11-26 , DOI: 10.1021/acsearthspacechem.9b00278 Alain Manceau 1 , Kathryn L. Nagy 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-11-26 , DOI: 10.1021/acsearthspacechem.9b00278 Alain Manceau 1 , Kathryn L. Nagy 2
Affiliation
|
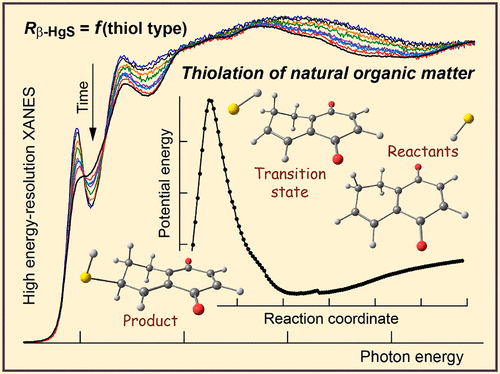
Thiol functional groups in natural organic matter form strong complexes with Hg(II) and other soft metal cations and therefore are an important component of sulfur and metal cycling in the environment. However, characterizing thiol reactivity is difficult because natural organic molecules are complex both in composition and molecular structure. Here, reactivity was assessed by calculating the Gibbs free energies of thiolation and thiol deprotonation reactions for model structures considered to form during abiotic sulfurization of natural organic matter. Gaussian calculations were performed at the CCSD(T) level of theory. Thiol addition is predicted to be faster by as much as 8 orders of magnitude on unsaturated cyclic structures rich in ketone and ether linkages than on open-chain carbonyl structures. The pKa values of thiols added to carboxyl-rich alicyclic molecules are predicted to be above 10, whereas pKa values of thiols bonded to lignin-derived polyhydroxyphenols are predicted to be between 2 and 6. Reactivity of thiols with Hg(II) was evaluated by monitoring the kinetics of transformation of Hg(SR)2 complexes to nanoparticulate metacinnabar in dissolved organic matter with different chemical compositions under oxic conditions using high-energy-resolution X-ray absorption near-edge structure spectroscopy (HR-XANES). The most rapid transformation occurred in the material with the greatest quinone carbon (ketone) and polyphenol contents, consistent with the predicted low pKa values of thiolated ketones near phenolic hydroxyl groups. The slowest transformation occurred in the material with the least amount of cyclic ketone structures despite having the highest amount of thiols. These results indicate that the sulfur atoms in thiolated ketones are those that are most reactive and removed during the alkyl transfer reaction considered to nucleate metacinnabar and that the concentration of the low-pKa thiols controls the transformation rate.
中文翻译:

天然有机物中的硫醇:分子原理,酸度和与汞(II)的反应性,来自第一性原理计算和高能分辨X射线吸收近边缘结构光谱
天然有机物中的硫醇官能团与Hg(II)和其他软金属阳离子形成强络合物,因此是环境中硫和金属循环的重要成分。然而,由于天然有机分子在组成和分子结构上都是复杂的,表征硫醇反应性是困难的。在这里,通过计算被认为在天然有机物非生物硫化过程中形成的模型结构的巯基化和巯基去质子化反应的吉布斯自由能来评估反应性。高斯计算是在CCSD(T)的理论水平上进行的。据预测,在富含酮和醚键的不饱和环状结构上,硫醇的添加速度比在开链羰基结构上的快达8个数量级。在P ķ一添加到富含羧基的脂环族分子上的硫醇的Pb值预计在10以上,而与木质素衍生的多羟基酚结合的硫醇的p K a值在2到6之间。使用高能量分辨率X射线吸收近边缘结构光谱(HR-XANES)监测在含氧条件下具有不同化学组成的Hg(SR)2复合物转化为溶解的有机物质中的纳米颗粒间朱砂的动力学。最快的转变发生在醌碳(酮)和多酚含量最高的材料中,与预测的低p K a相一致酚羟基附近的硫醇化酮的分子量值 尽管具有最高量的硫醇,但最慢的转化发生在具有最少数量的环状酮结构的材料中。这些结果表明,在被认为使间朱砂成核的烷基转移反应中,硫醇化酮中的硫原子是最具反应性的硫原子,并且低p K a硫醇的浓度控制了转化率。
更新日期:2019-11-28
中文翻译:

天然有机物中的硫醇:分子原理,酸度和与汞(II)的反应性,来自第一性原理计算和高能分辨X射线吸收近边缘结构光谱
天然有机物中的硫醇官能团与Hg(II)和其他软金属阳离子形成强络合物,因此是环境中硫和金属循环的重要成分。然而,由于天然有机分子在组成和分子结构上都是复杂的,表征硫醇反应性是困难的。在这里,通过计算被认为在天然有机物非生物硫化过程中形成的模型结构的巯基化和巯基去质子化反应的吉布斯自由能来评估反应性。高斯计算是在CCSD(T)的理论水平上进行的。据预测,在富含酮和醚键的不饱和环状结构上,硫醇的添加速度比在开链羰基结构上的快达8个数量级。在P ķ一添加到富含羧基的脂环族分子上的硫醇的Pb值预计在10以上,而与木质素衍生的多羟基酚结合的硫醇的p K a值在2到6之间。使用高能量分辨率X射线吸收近边缘结构光谱(HR-XANES)监测在含氧条件下具有不同化学组成的Hg(SR)2复合物转化为溶解的有机物质中的纳米颗粒间朱砂的动力学。最快的转变发生在醌碳(酮)和多酚含量最高的材料中,与预测的低p K a相一致酚羟基附近的硫醇化酮的分子量值 尽管具有最高量的硫醇,但最慢的转化发生在具有最少数量的环状酮结构的材料中。这些结果表明,在被认为使间朱砂成核的烷基转移反应中,硫醇化酮中的硫原子是最具反应性的硫原子,并且低p K a硫醇的浓度控制了转化率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号