当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cloud Water Chemistry Associated with Urban Aerosols: Rapid Hydroxyl Radical Formation, Soluble Metals, Fe(II), Fe(III), and Quinones
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-12-17 , DOI: 10.1021/acsearthspacechem.9b00243 Xiaobi M. Kuang 1 , David H. Gonzalez 1 , J. Adlin Scott 1 , Kennedy Vu 2 , Alam Hasson 2 , Tiffany Charbouillot 1 , Lelia Hawkins 3 , Suzanne E. Paulson 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-12-17 , DOI: 10.1021/acsearthspacechem.9b00243 Xiaobi M. Kuang 1 , David H. Gonzalez 1 , J. Adlin Scott 1 , Kennedy Vu 2 , Alam Hasson 2 , Tiffany Charbouillot 1 , Lelia Hawkins 3 , Suzanne E. Paulson 1
Affiliation
|
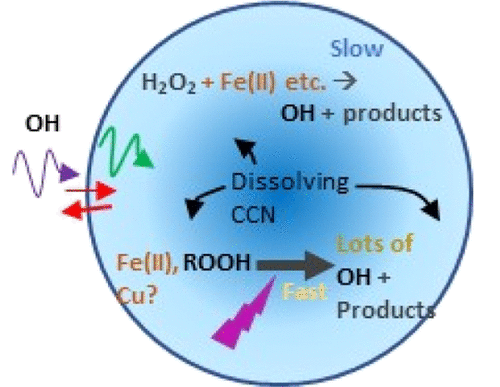
Hydroxyl radical (OH) reactions in cloud water play a key role in secondary organic aerosol formation and sulfur oxidation. We collected aerosol samples (PM4) during summer at an urban receptor site in Southern California. The site mostly receives air from the urban area in the morning, photochemically processed air arriving from the urban area and a commercial ports area in the afternoon, and a largely unpopulated mountainous area overnight. Filters were extracted in small quantities of water at pH 3.5 (adjusted with H2SO4), simulating cloud water formation. Samples were analyzed for particle mass, OH generation in the presence of near UV light, soluble trace metals (filtered through a 0.22 μm filter, measured with inductively coupled plasma mass spectrometry, ICP–MS), soluble Fe(II) and Fe(III) (measured with the ferrozine assay, Fefzn), and quinones. Soluble speciated iron was about equally divided into Fe(II)fzn and Fe(III)fzn and accounted for only 22 ± 7% of the soluble Fe measured with ICP–MS. The highest concentrations of Fefzn came from the urban area; high FeICP came both from city and mountains, and the mountains were the dominant source of Cu. OH formation was characterized by an initial spike in formation lasting for 1–3 min with a formation rate at ∼(0.2–1) × 10–8 M·s–1, followed by a second much slower phase of (0.1–10) × 10–11 M·s–1. OH formation activity was strongly correlated with mass and soluble (ICP) Fe and Cu; it did not correlate with ferrozine iron. Quinone concentrations were too low to contribute much to OH formation. The initial burst of OH formation is large enough to contribute substantial OH to cloud and fog droplets.
中文翻译:

与城市气溶胶有关的云水化学:快速形成羟基自由基,可溶性金属,Fe(II),Fe(III)和醌
云水中的羟基自由基(OH)反应在二次有机气溶胶的形成和硫的氧化中起关键作用。夏季,我们在南加州的一个城市接收站收集了气溶胶样品(PM 4)。该站点大部分早上从市区接收空气,下午从市区和商业港口区接收经过光化学处理的空气,一夜之间则从人口稀少的山区接收。滤纸用少量pH 3.5的水萃取(用H 2 SO 4调节)),模拟云水的形成。分析了样品的颗粒质量,在近紫外光下产生的OH,可溶性痕量金属(通过0.22μm过滤器过滤,通过电感耦合等离子体质谱法,ICP-MS测量),可溶性Fe(II)和Fe(III )(用铁试剂测定,Fe fzn)和醌。可溶性特种铁大致分为Fe(II)fzn和Fe(III)fzn,仅占ICP-MS测定的可溶性Fe的22±7%。Fe fzn的最高浓度来自市区。高铁ICP来自城市和山区,山区是铜的主要来源。OH形成的特征是,初始形成峰持续1–3分钟,形成速率约为(0.2–1)×10 –8 M·s –1,然后是第二个慢得多的阶段(0.1–10) ×10 –11 M·s –1。OH的形成活性与铁和铜的质量和可溶性(ICP)密切相关。它与ferrozine铁不相关。醌的浓度太低,不足以促进OH的形成。OH形成的初始爆发足够大,足以向云雾和雾滴中贡献大量OH。
更新日期:2019-12-18
中文翻译:

与城市气溶胶有关的云水化学:快速形成羟基自由基,可溶性金属,Fe(II),Fe(III)和醌
云水中的羟基自由基(OH)反应在二次有机气溶胶的形成和硫的氧化中起关键作用。夏季,我们在南加州的一个城市接收站收集了气溶胶样品(PM 4)。该站点大部分早上从市区接收空气,下午从市区和商业港口区接收经过光化学处理的空气,一夜之间则从人口稀少的山区接收。滤纸用少量pH 3.5的水萃取(用H 2 SO 4调节)),模拟云水的形成。分析了样品的颗粒质量,在近紫外光下产生的OH,可溶性痕量金属(通过0.22μm过滤器过滤,通过电感耦合等离子体质谱法,ICP-MS测量),可溶性Fe(II)和Fe(III )(用铁试剂测定,Fe fzn)和醌。可溶性特种铁大致分为Fe(II)fzn和Fe(III)fzn,仅占ICP-MS测定的可溶性Fe的22±7%。Fe fzn的最高浓度来自市区。高铁ICP来自城市和山区,山区是铜的主要来源。OH形成的特征是,初始形成峰持续1–3分钟,形成速率约为(0.2–1)×10 –8 M·s –1,然后是第二个慢得多的阶段(0.1–10) ×10 –11 M·s –1。OH的形成活性与铁和铜的质量和可溶性(ICP)密切相关。它与ferrozine铁不相关。醌的浓度太低,不足以促进OH的形成。OH形成的初始爆发足够大,足以向云雾和雾滴中贡献大量OH。











































 京公网安备 11010802027423号
京公网安备 11010802027423号