Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prescription of benzodiazepines, z-drugs, and gabapentinoids and mortality risk in people receiving opioid agonist treatment: Observational study based on the UK Clinical Practice Research Datalink and Office for National Statistics death records.
PLOS Medicine ( IF 10.5 ) Pub Date : 2019-11-26 , DOI: 10.1371/journal.pmed.1002965 John Macleod 1 , Colin Steer 1 , Kate Tilling 1 , Rosie Cornish 1 , John Marsden 2 , Tim Millar 3 , John Strang 2 , Matthew Hickman 1
PLOS Medicine ( IF 10.5 ) Pub Date : 2019-11-26 , DOI: 10.1371/journal.pmed.1002965 John Macleod 1 , Colin Steer 1 , Kate Tilling 1 , Rosie Cornish 1 , John Marsden 2 , Tim Millar 3 , John Strang 2 , Matthew Hickman 1
Affiliation
|
BACKGROUND
Patients with opioid dependency prescribed opioid agonist treatment (OAT) may also be prescribed sedative drugs. This may increase mortality risk but may also increase treatment duration, with overall benefit. We hypothesised that prescription of benzodiazepines in patients receiving OAT would increase risk of mortality overall, irrespective of any increased treatment duration.
METHODS AND FINDINGS
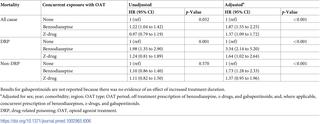
Data on 12,118 patients aged 15-64 years prescribed OAT between 1998 and 2014 were extracted from the Clinical Practice Research Datalink. Data from the Office for National Statistics on whether patients had died and, if so, their cause of death were available for 7,016 of these patients. We identified episodes of prescription of benzodiazepines, z-drugs, and gabapentinoids and used linear regression and Cox proportional hazards models to assess the associations of co-prescription (prescribed during OAT and up to 12 months post-treatment) and concurrent prescription (prescribed during OAT) with treatment duration and mortality. We examined all-cause mortality (ACM), drug-related poisoning (DRP) mortality, and mortality not attributable to DRP (non-DRP). Models included potential confounding factors. In 36,126 person-years of follow-up there were 657 deaths and 29,540 OAT episodes, of which 42% involved benzodiazepine co-prescription and 29% concurrent prescription (for z-drugs these respective proportions were 20% and 11%, and for gabapentinoids 8% and 5%). Concurrent prescription of benzodiazepines was associated with increased duration of methadone treatment (adjusted mean duration of treatment episode 466 days [95% CI 450 to 483] compared to 286 days [95% CI 275 to 297]). Benzodiazepine co-prescription was associated with increased risk of DRP (adjusted HR 2.96 [95% CI 1.97 to 4.43], p < 0.001), with evidence of a dose-response effect, but showed little evidence of an association with non-DRP (adjusted HR 0.91 [95% CI 0.66 to 1.25], p = 0.549). Co-prescription of z-drugs showed evidence of an association with increased risk of DRP (adjusted HR 2.75 [95% CI 1.57 to 4.83], p < 0.001) but little evidence of an association with non-DRP (adjusted HR 0.79 [95% CI 0.49 to 1.28], p = 0.342). There was no evidence of an association of gabapentinoid co-prescription with DRP (HR 1.54 [95% CI 0.60 to 3.98], p = 0.373) but evidence of an association with increased non-DRP (HR 1.83 [95% CI 1.28 to 2.62], p = 0.001). Concurrent benzodiazepine prescription also increased mortality risk after consideration of duration of OAT (adjusted HR for DRP with benzodiazepine concurrent prescription 3.34 [95% CI 2.14 to 5.20], p < 0.001). The main limitation of this study is the possibility that unmeasured confounding factors led to an association between benzodiazepine prescription and DRP that is not causal.
CONCLUSIONS
In this study, co-prescription of benzodiazepine was specifically associated with increased risk of DRP in opioid-dependent individuals. Co-prescription of z-drugs and gabapentinoids was also associated with increased mortality risk; however, for z-drugs there was no evidence for a dose-response effect on DRP, and for gabapentinoids the increased mortality risk was not specific to DRP. Concurrent prescription of benzodiazepine was associated with longer treatment but still increased risk of death overall. Clinicians should be cautious about prescribing benzodiazepines to opioid-dependent individuals.
中文翻译:

接受阿片类激动剂治疗的人的苯二氮卓类药物,z-药物和加巴喷丁胺的处方及死亡风险:基于英国临床实践研究数据链和国家统计局死亡记录的观察性研究。
背景技术有阿片类药物依赖的患者处方了阿片类药物激动剂治疗(OAT),也可以开具镇静药。这可能会增加死亡风险,但也可能会延长治疗时间,并带来总体收益。我们假设接受OAT治疗的患者服用苯二氮卓类药物会增加整体的死亡风险,而与治疗时间的延长无关。方法和研究结果从临床实践研究数据链接中提取了1998年至2014年之间12118例15-64岁的OAT处方患者的数据。国家统计局提供的有关患者是否死亡以及死亡原因的数据可供这些患者中的7,016名患者使用。我们确定了苯二氮卓类药物,z-药物,和加巴喷丁类药物,并使用线性回归和Cox比例风险模型评估联合处方(在OAT期间和治疗后长达12个月的处方)和同时处方(在OAT期间的处方)与治疗持续时间和死亡率之间的关联。我们检查了全因死亡率(ACM),药物相关中毒(DRP)死亡率以及非DRP引起的死亡率(non-DRP)。模型包括潜在的混杂因素。在36,126人年的随访中,有657例死亡和29,540例OAT发作,其中42%涉及苯二氮卓联合处方和29%并发处方(对于z药物,这两个比例分别为20%和11%,对于加巴喷丁类化合物8%和5%)。并用苯二氮卓类药物与美沙酮治疗的持续时间增加相关(调整后的平均治疗持续时间为466天[95%CI 450至483],而286天[95%CI 275至297])。苯二氮卓共同处方与DRP风险增加相关(校正后的HR 2.96 [95%CI 1.97至4.43],p <0.001),具有剂量反应作用的证据,但几乎没有证据表明与非DRP相关(调整后的HR 0.91 [95%CI 0.66至1.25],p = 0.549)。Z药物的共同处方显示有证据表明与DRP风险增加相关(校正后的HR 2.75 [95%CI 1.57至4.83],p <0.001),但几乎没有证据表明与非DRP相关(校正的HR 0.79 [95] %CI 0.49至1.28],p = 0.342)。没有证据表明加巴喷丁类处方药与DRP有关联(HR 1.54 [95%CI 0.60至3。98],p = 0.373),但与非DRP增加相关的证据(HR 1.83 [95%CI 1.28至2.62],p = 0.001)。在考虑OAT持续时间后,并用苯二氮卓类药物的处方也增加了死亡风险(将DRP与苯并二氮杂胺并发的处方药物的HR调整为3.34 [95%CI 2.14至5.20],p <0.001)。这项研究的主要局限性是不可测的混杂因素可能导致苯二氮卓处方与DRP之间的联系不是因果关系。结论在这项研究中,苯二氮卓类药物的共同处方与阿片类药物依赖性个体的DRP风险增加特别相关。共同处方z药物和加巴喷丁类药物也可能增加死亡率。但是,对于z药物,没有证据表明对DRP有剂量反应作用,对于加巴喷丁类药物而言,增加的死亡风险并不是DRP所特有的。并用苯二氮卓类药物可延长治疗时间,但总体上仍增加死亡风险。临床医生在向依赖阿片类药物的人开具苯二氮卓类药物时应保持谨慎。
更新日期:2019-12-03
中文翻译:

接受阿片类激动剂治疗的人的苯二氮卓类药物,z-药物和加巴喷丁胺的处方及死亡风险:基于英国临床实践研究数据链和国家统计局死亡记录的观察性研究。
背景技术有阿片类药物依赖的患者处方了阿片类药物激动剂治疗(OAT),也可以开具镇静药。这可能会增加死亡风险,但也可能会延长治疗时间,并带来总体收益。我们假设接受OAT治疗的患者服用苯二氮卓类药物会增加整体的死亡风险,而与治疗时间的延长无关。方法和研究结果从临床实践研究数据链接中提取了1998年至2014年之间12118例15-64岁的OAT处方患者的数据。国家统计局提供的有关患者是否死亡以及死亡原因的数据可供这些患者中的7,016名患者使用。我们确定了苯二氮卓类药物,z-药物,和加巴喷丁类药物,并使用线性回归和Cox比例风险模型评估联合处方(在OAT期间和治疗后长达12个月的处方)和同时处方(在OAT期间的处方)与治疗持续时间和死亡率之间的关联。我们检查了全因死亡率(ACM),药物相关中毒(DRP)死亡率以及非DRP引起的死亡率(non-DRP)。模型包括潜在的混杂因素。在36,126人年的随访中,有657例死亡和29,540例OAT发作,其中42%涉及苯二氮卓联合处方和29%并发处方(对于z药物,这两个比例分别为20%和11%,对于加巴喷丁类化合物8%和5%)。并用苯二氮卓类药物与美沙酮治疗的持续时间增加相关(调整后的平均治疗持续时间为466天[95%CI 450至483],而286天[95%CI 275至297])。苯二氮卓共同处方与DRP风险增加相关(校正后的HR 2.96 [95%CI 1.97至4.43],p <0.001),具有剂量反应作用的证据,但几乎没有证据表明与非DRP相关(调整后的HR 0.91 [95%CI 0.66至1.25],p = 0.549)。Z药物的共同处方显示有证据表明与DRP风险增加相关(校正后的HR 2.75 [95%CI 1.57至4.83],p <0.001),但几乎没有证据表明与非DRP相关(校正的HR 0.79 [95] %CI 0.49至1.28],p = 0.342)。没有证据表明加巴喷丁类处方药与DRP有关联(HR 1.54 [95%CI 0.60至3。98],p = 0.373),但与非DRP增加相关的证据(HR 1.83 [95%CI 1.28至2.62],p = 0.001)。在考虑OAT持续时间后,并用苯二氮卓类药物的处方也增加了死亡风险(将DRP与苯并二氮杂胺并发的处方药物的HR调整为3.34 [95%CI 2.14至5.20],p <0.001)。这项研究的主要局限性是不可测的混杂因素可能导致苯二氮卓处方与DRP之间的联系不是因果关系。结论在这项研究中,苯二氮卓类药物的共同处方与阿片类药物依赖性个体的DRP风险增加特别相关。共同处方z药物和加巴喷丁类药物也可能增加死亡率。但是,对于z药物,没有证据表明对DRP有剂量反应作用,对于加巴喷丁类药物而言,增加的死亡风险并不是DRP所特有的。并用苯二氮卓类药物可延长治疗时间,但总体上仍增加死亡风险。临床医生在向依赖阿片类药物的人开具苯二氮卓类药物时应保持谨慎。











































 京公网安备 11010802027423号
京公网安备 11010802027423号