当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disrupting the Conserved Salt Bridge in the Trimerization of Influenza A Nucleoprotein.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.jmedchem.9b01244 Jennifer L Woodring,Shao-Hung Lu,Larissa Krasnova,Shih-Chi Wang,Jhih-Bin Chen,Chiu-Chun Chou,Yi-Chou Huang,Ting-Jen Rachel Cheng,Ying-Ta Wu,Yu-Hou Chen,Jim-Min Fang,Ming-Daw Tsai,Chi-Huey Wong
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.jmedchem.9b01244 Jennifer L Woodring,Shao-Hung Lu,Larissa Krasnova,Shih-Chi Wang,Jhih-Bin Chen,Chiu-Chun Chou,Yi-Chou Huang,Ting-Jen Rachel Cheng,Ying-Ta Wu,Yu-Hou Chen,Jim-Min Fang,Ming-Daw Tsai,Chi-Huey Wong
|
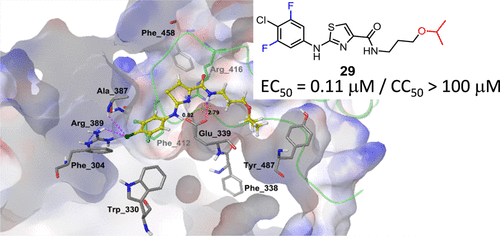
Antiviral drug resistance in influenza infections has been a major threat to public health. To develop a broad-spectrum inhibitor of influenza to combat the problem of drug resistance, we previously identified the highly conserved E339...R416 salt bridge of the nucleoprotein trimer as a target and compound 1 as an inhibitor disrupting the salt bridge with an EC50 = 2.7 μM against influenza A (A/WSN/1933). We have further modified this compound via a structure-based approach and performed antiviral activity screening to identify compounds 29 and 30 with EC50 values of 110 and 120 nM, respectively, and without measurable host cell cytotoxicity. Compared to the clinically used neuraminidase inhibitors, these two compounds showed better activity profiles against drug-resistant influenza A strains, as well as influenza B, and improved survival of influenza-infected mice.
中文翻译:

在甲型流感病毒核蛋白三聚化过程中破坏保守的盐桥。
流感感染中的抗病毒药物耐药性已成为对公共卫生的主要威胁。为了开发广谱的流感抑制剂来解决耐药性问题,我们之前确定了高度保守的核蛋白三聚体E339 ... R416盐桥为靶标,化合物1为通过EC50破坏盐桥的抑制剂甲型流感病毒= 2.7μM(A / WSN / 1933)。我们通过基于结构的方法进一步修饰了该化合物,并进行了抗病毒活性筛选,以鉴定化合物29和30的EC50值分别为110和120 nM,并且没有可测量的宿主细胞毒性。与临床使用的神经氨酸酶抑制剂相比,这两种化合物对耐药性A型流感病毒株和B型流感病毒显示出更好的活性,
更新日期:2019-12-18
中文翻译:

在甲型流感病毒核蛋白三聚化过程中破坏保守的盐桥。
流感感染中的抗病毒药物耐药性已成为对公共卫生的主要威胁。为了开发广谱的流感抑制剂来解决耐药性问题,我们之前确定了高度保守的核蛋白三聚体E339 ... R416盐桥为靶标,化合物1为通过EC50破坏盐桥的抑制剂甲型流感病毒= 2.7μM(A / WSN / 1933)。我们通过基于结构的方法进一步修饰了该化合物,并进行了抗病毒活性筛选,以鉴定化合物29和30的EC50值分别为110和120 nM,并且没有可测量的宿主细胞毒性。与临床使用的神经氨酸酶抑制剂相比,这两种化合物对耐药性A型流感病毒株和B型流感病毒显示出更好的活性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号