当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Mechanisms of the Ultra-Strong Inhibition Effect of Oxidized Carbon Dots on Human Insulin Fibrillation
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-01-08 , DOI: 10.1021/acsabm.9b00725 Qi-Qi Yang 1 , Huan He 1 , Chen-Qiao Li 1 , Lai-Bing Luo 1 , Shu-Lan Li 1 , Zi-Qiang Xu 2 , Jian-Cheng Jin 1 , Feng-Lei Jiang 1 , Yi Liu 1, 3, 4 , Mian Yang 4
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-01-08 , DOI: 10.1021/acsabm.9b00725 Qi-Qi Yang 1 , Huan He 1 , Chen-Qiao Li 1 , Lai-Bing Luo 1 , Shu-Lan Li 1 , Zi-Qiang Xu 2 , Jian-Cheng Jin 1 , Feng-Lei Jiang 1 , Yi Liu 1, 3, 4 , Mian Yang 4
Affiliation
|
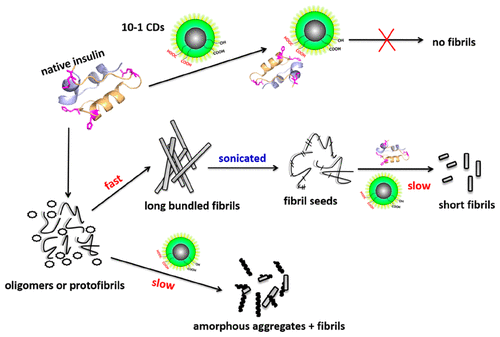
Amyloid fibrillation of protein is associated with a great variety of pathologic conditions. The aggregation of protein is a complicated process with multisteps, whereas most of the inhibitors with elaborately designed structures can show an inhibition effect only on the nucleation stages of protein fibrillation. Herein, oxidized carbon dots (CDs) were achieved to study the relationship between the surface properties of CDs and their inhibition effect on human insulin (HI) fibrillation. More oxygen-containing function groups can be obtained after oxidation reaction of CDs, such as −OH and −COOH. The results show that 10–1 CDs (the mass ratios of CD/KMnO4 is 10:1), with the highest carboxyl group content, possess the best inhibition ability. All the nucleation, growth, and final phases can be retarded by 10–1 CDs, which have been studied in detail by fluorescence spectra. However, CDs without oxidation can show only a weak inhibition effect on the nucleation stage. The 10–1 CDs is demonstrated to binding with HI monomers much stronger than that of CDs by isothermal titration calorimetry (ITC). Moreover, molecular dynamics simulations (MD) studies indicate that CDs with more carboxyl groups can show stronger affinities with native or unfolded HI monomers, which may be mainly derived from the active binding sites of histidine residues (His5 and His10) on B-chain through electrostatic interaction. Because the unfolding of B-chain in HI is prior to that of A-chain in our MD simulations, the later aggregation of HI can be inhibited effectively by the stronger binding forces between 10 and 1 CDs and the B-chain of HI.
中文翻译:

氧化碳点对人胰岛素纤颤的超强抑制作用的分子机制
蛋白质的淀粉样蛋白纤颤与多种病理状况有关。蛋白质的聚集是一个多步骤的复杂过程,而大多数精心设计的结构抑制剂只能在蛋白质原纤化的成核阶段发挥抑制作用。在此,实现了氧化碳点 (CDs) 以研究 CDs 的表面性质与其对人胰岛素 (HI) 纤颤的抑制作用之间的关系。CDs经过氧化反应可以得到更多的含氧官能团,如-OH和-COOH。结果表明,10-1 个 CDs(CD/KMnO 4的质量比10:1),羧基含量最高,抑制能力最好。所有的成核、生长和最终阶段都可以被 10-1 个 CD 延迟,这已通过荧光光谱进行了详细研究。然而,没有氧化的CDs在成核阶段只能表现出微弱的抑制作用。等温滴定量热法 (ITC) 证明 10-1 CD 与 HI 单体的结合比 CD 强得多。此外,分子动力学模拟 (MD) 研究表明,具有更多羧基的 CD 可以与天然或未折叠的 HI 单体表现出更强的亲和力,这可能主要来源于 B 链上组氨酸残基 (His5 和 His10) 的活性结合位点。静电相互作用。因为在我们的 MD 模拟中,HI 中 B 链的展开先于 A 链的展开,
更新日期:2020-01-08
中文翻译:

氧化碳点对人胰岛素纤颤的超强抑制作用的分子机制
蛋白质的淀粉样蛋白纤颤与多种病理状况有关。蛋白质的聚集是一个多步骤的复杂过程,而大多数精心设计的结构抑制剂只能在蛋白质原纤化的成核阶段发挥抑制作用。在此,实现了氧化碳点 (CDs) 以研究 CDs 的表面性质与其对人胰岛素 (HI) 纤颤的抑制作用之间的关系。CDs经过氧化反应可以得到更多的含氧官能团,如-OH和-COOH。结果表明,10-1 个 CDs(CD/KMnO 4的质量比10:1),羧基含量最高,抑制能力最好。所有的成核、生长和最终阶段都可以被 10-1 个 CD 延迟,这已通过荧光光谱进行了详细研究。然而,没有氧化的CDs在成核阶段只能表现出微弱的抑制作用。等温滴定量热法 (ITC) 证明 10-1 CD 与 HI 单体的结合比 CD 强得多。此外,分子动力学模拟 (MD) 研究表明,具有更多羧基的 CD 可以与天然或未折叠的 HI 单体表现出更强的亲和力,这可能主要来源于 B 链上组氨酸残基 (His5 和 His10) 的活性结合位点。静电相互作用。因为在我们的 MD 模拟中,HI 中 B 链的展开先于 A 链的展开,











































 京公网安备 11010802027423号
京公网安备 11010802027423号