当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery and Evaluation of Peptide Ligands for Selective Adsorption and Release of Cas9 Nuclease on Solid Substrates.
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2019-12-09 , DOI: 10.1021/acs.bioconjchem.9b00703 Kevin Day 1 , Raphael Prodromou 1 , Sahand Saberi Bosari 1 , Ashton Lavoie 1 , Mohammad Omary 1 , Connor Market 1 , Adriana San Miguel 1 , Stefano Menegatti 1, 2
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2019-12-09 , DOI: 10.1021/acs.bioconjchem.9b00703 Kevin Day 1 , Raphael Prodromou 1 , Sahand Saberi Bosari 1 , Ashton Lavoie 1 , Mohammad Omary 1 , Connor Market 1 , Adriana San Miguel 1 , Stefano Menegatti 1, 2
Affiliation
|
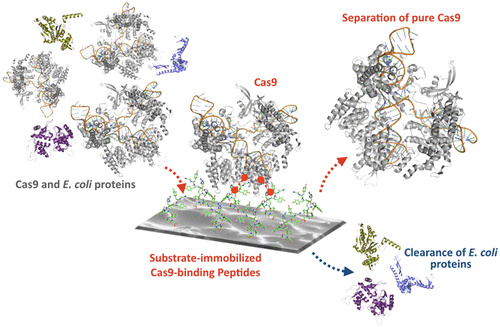
The rapid expansion of CRISPR in biotechnology, medicine, and bioprocessing poses an urgent need for advanced manufacturing of Cas nucleases. The lack of Cas-targeting ligands, however, prevents the development of platform processes for purifying this class of molecules. This work represents the first effort at developing short synthetic Cas9-binding peptides and demonstrates their applicability as affinity ligands for the purification of a Cas nuclease. Candidate Cas9-targeting peptides were initially identified by screening a solid-phase peptide library against a model mixture of Streptococcus pyogenes Cas9 spiked in Escherichia coli cell lysate. An ensemble of homologous sequences was identified, conjugated on Toyopearl resin, and evaluated by Cas9 binding studies to identify sequences providing selective Cas9 capture and efficient release. In silico docking studies were also performed to evaluate the binding energy and site of the various peptides on Cas9. Notably, sequences GYYRYSEY and YYHRHGLQ were shown to target the RecII domain of Cas9, which is not involved in nuclease activity and was targeted as an ideal binding site. The peptide ligands were validated by purifying Cas9 from the E. coli lysate in dynamic conditions and through measurements of binding capacity and strength (Qmax and KD). The resulting values of Qmax = 4-5 mg Cas9 per mL of resin and KD ∼ 0.1-0.3 μM, product recovery (86-89%), and purity (91%-93%) indicate that both peptides, and YYHRHGLQ in particular, can serve as capture ligands in a platform purification process of Cas9.
中文翻译:

发现和评估用于在固体基质上选择性吸附和释放Cas9核酸酶的肽配体。
CRISPR在生物技术,医学和生物加工领域的快速发展,迫切需要对Cas核酸酶进行先进的生产。但是,缺乏靶向Cas的配体会阻止开发用于纯化此类分子的平台方法。这项工作代表了开发短的合成Cas9结合肽的第一步,并证明了它们可作为亲和配体用于纯化Cas核酸酶。通过针对掺入大肠杆菌细胞裂解物中的化脓性链球菌Cas9的模型混合物筛选固相肽库,初步鉴定了靶向Cas9的候选肽。鉴定出一组同源序列,缀合在Toyopearl树脂上,并通过Cas9结合研究进行评估,以鉴定提供选择性Cas9捕获和有效释放的序列。还进行了计算机对接研究,以评估Cas9上各种肽的结合能和位点。值得注意的是,序列GYYRYSEY和YYHRHGLQ显示出靶向Cas9的RecII结构域,其不参与核酸酶活性并且被靶向为理想的结合位点。通过在动态条件下从大肠杆菌裂解物中纯化Cas9并通过测量结合能力和强度(Qmax和KD)来验证肽配体。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。已显示序列GYYRYSEY和YYHRHGLQ靶向Cas9的RecII结构域,其不参与核酸酶活性并且被靶向作为理想的结合位点。通过在动态条件下从大肠杆菌裂解物中纯化Cas9并通过测量结合能力和强度(Qmax和KD)来验证肽配体。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。已显示序列GYYRYSEY和YYHRHGLQ靶向Cas9的RecII结构域,其不参与核酸酶活性并且被靶向作为理想的结合位点。通过在动态条件下从大肠杆菌裂解物中纯化Cas9并通过测量结合能力和强度(Qmax和KD)来验证肽配体。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。大肠杆菌裂解物在动态条件下并通过测量结合能力和强度(Qmax和KD)。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。大肠杆菌裂解物在动态条件下并通过测量结合能力和强度(Qmax和KD)。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。
更新日期:2019-12-11
中文翻译:

发现和评估用于在固体基质上选择性吸附和释放Cas9核酸酶的肽配体。
CRISPR在生物技术,医学和生物加工领域的快速发展,迫切需要对Cas核酸酶进行先进的生产。但是,缺乏靶向Cas的配体会阻止开发用于纯化此类分子的平台方法。这项工作代表了开发短的合成Cas9结合肽的第一步,并证明了它们可作为亲和配体用于纯化Cas核酸酶。通过针对掺入大肠杆菌细胞裂解物中的化脓性链球菌Cas9的模型混合物筛选固相肽库,初步鉴定了靶向Cas9的候选肽。鉴定出一组同源序列,缀合在Toyopearl树脂上,并通过Cas9结合研究进行评估,以鉴定提供选择性Cas9捕获和有效释放的序列。还进行了计算机对接研究,以评估Cas9上各种肽的结合能和位点。值得注意的是,序列GYYRYSEY和YYHRHGLQ显示出靶向Cas9的RecII结构域,其不参与核酸酶活性并且被靶向为理想的结合位点。通过在动态条件下从大肠杆菌裂解物中纯化Cas9并通过测量结合能力和强度(Qmax和KD)来验证肽配体。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。已显示序列GYYRYSEY和YYHRHGLQ靶向Cas9的RecII结构域,其不参与核酸酶活性并且被靶向作为理想的结合位点。通过在动态条件下从大肠杆菌裂解物中纯化Cas9并通过测量结合能力和强度(Qmax和KD)来验证肽配体。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。已显示序列GYYRYSEY和YYHRHGLQ靶向Cas9的RecII结构域,其不参与核酸酶活性并且被靶向作为理想的结合位点。通过在动态条件下从大肠杆菌裂解物中纯化Cas9并通过测量结合能力和强度(Qmax和KD)来验证肽配体。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。大肠杆菌裂解物在动态条件下并通过测量结合能力和强度(Qmax和KD)。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。大肠杆菌裂解物在动态条件下并通过测量结合能力和强度(Qmax和KD)。Qmax = 4-5 mg Cas9 / mL树脂和KD约0.1-0.3μM,产物回收率(86-89%)和纯度(91%-93%)的结果表明这两种肽尤其是YYHRHGLQ可以在Cas9的平台纯化过程中用作捕获配体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号