当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical intramolecular C–H/N–H functionalization for the synthesis of isoxazolidine-fused isoquinolin-1(2H)-ones
Green Chemistry ( IF 9.8 ) Pub Date : 2019-11-26 , DOI: 10.1039/c9gc03290h Lin-Bao Zhang 1, 2, 3, 4, 5 , Rui-Sen Geng 1, 2, 3, 4, 5 , Zi-Chen Wang 1, 2, 3, 4, 5 , Guang-Yi Ren 1, 2, 3, 4, 5 , Li-Rong Wen 1, 2, 3, 4, 5 , Ming Li 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2019-11-26 , DOI: 10.1039/c9gc03290h Lin-Bao Zhang 1, 2, 3, 4, 5 , Rui-Sen Geng 1, 2, 3, 4, 5 , Zi-Chen Wang 1, 2, 3, 4, 5 , Guang-Yi Ren 1, 2, 3, 4, 5 , Li-Rong Wen 1, 2, 3, 4, 5 , Ming Li 1, 2, 3, 4, 5
Affiliation
|
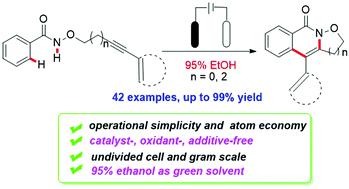
A general and practical protocol for the construction of isoxazolidine-fused isoquinolin-1(2H)-ones has been described by electrochemical-oxidation-induced intramolecular annulation via amidyl radicals. In an undivided cell, isoquinolinones could be easily generated from various available amides bearing CONHOR groups under metal-free, additive-free and external oxidant-free conditions. Moreover, this transformation proceeded smoothly by using cheap 95% ethanol as the green solvent and could be extended to the gram scale.
中文翻译:

电化学分子内C–H / N–H官能团用于合成异恶唑烷融合的异喹啉-1(2H)-一
已经通过电化学氧化诱导的经由酰胺基的分子内环化描述了用于构建异恶唑烷融合的异喹啉-1(2 H)-one的通用和实用的方案。在一个没有分隔的细胞中,异喹啉酮很容易在无金属,无添加剂和无外部氧化剂的条件下从带有CONHOR基团的各种可用酰胺中生成。此外,通过使用廉价的95%乙醇作为绿色溶剂,该转化过程可以顺利进行,并且可以扩展到克级。
更新日期:2019-11-26
中文翻译:

电化学分子内C–H / N–H官能团用于合成异恶唑烷融合的异喹啉-1(2H)-一
已经通过电化学氧化诱导的经由酰胺基的分子内环化描述了用于构建异恶唑烷融合的异喹啉-1(2 H)-one的通用和实用的方案。在一个没有分隔的细胞中,异喹啉酮很容易在无金属,无添加剂和无外部氧化剂的条件下从带有CONHOR基团的各种可用酰胺中生成。此外,通过使用廉价的95%乙醇作为绿色溶剂,该转化过程可以顺利进行,并且可以扩展到克级。



























 京公网安备 11010802027423号
京公网安备 11010802027423号