当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-Assembly and Neurotoxicity of β-Amyloid (21-40) Peptide Fragment: The Regulatory Role of GxxxG Motifs.
ChemMedChem ( IF 3.4 ) Pub Date : 2019-11-24 , DOI: 10.1002/cmdc.201900620 Dibakar Sarkar 1 , Ipsita Chakraborty 1 , Marcello Condorelli 2 , Baijayanti Ghosh 3 , Thorben Mass 4 , Markus Weingarth 4 , Atin K Mandal 3 , Carmelo La Rosa 2 , Vivekanandan Subramanian 5 , Anirban Bhunia 1
ChemMedChem ( IF 3.4 ) Pub Date : 2019-11-24 , DOI: 10.1002/cmdc.201900620 Dibakar Sarkar 1 , Ipsita Chakraborty 1 , Marcello Condorelli 2 , Baijayanti Ghosh 3 , Thorben Mass 4 , Markus Weingarth 4 , Atin K Mandal 3 , Carmelo La Rosa 2 , Vivekanandan Subramanian 5 , Anirban Bhunia 1
Affiliation
|
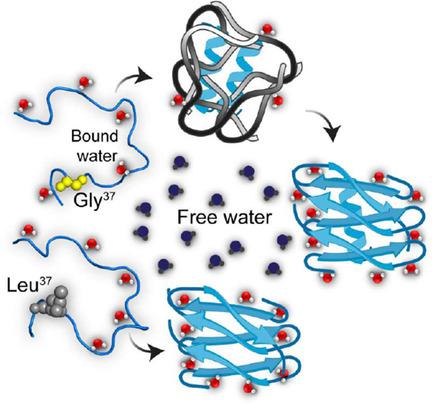
The three GxxxG repeating motifs from the C-terminal region of β-amyloid (Aβ) peptide play a significant role in regulating the aggregation kinetics of the peptide. Mutation of these glycine residues to leucine greatly accelerates the fibrillation process but generates a varied toxicity profile. Using an array of biophysical techniques, we demonstrated the uniqueness of the composite glycine residues in these structural repeats. We used solvent relaxation NMR spectroscopy to investigate the role played by the surrounding water molecules in determining the corresponding aggregation pathway. Notably, the conformational changes induced by Gly33 and Gly37 mutations result in significantly decreased toxicity in a neuronal cell line. Our results indicate that G33 xxxG37 is the primary motif responsible for Aβ neurotoxicity, hence providing a direct structure-function correlation. Targeting this motif, therefore, can be a promising strategy to prevent neuronal cell death associated with Alzheimer's and other related diseases, such as type II diabetes and Parkinson's.
中文翻译:

β-淀粉样蛋白(21-40)肽片段的自组装和神经毒性:GxxxG母题的调节作用。
来自β-淀粉样蛋白(Aβ)肽C端区域的三个GxxxG重复基序在调节该肽的聚集动力学中起重要作用。这些甘氨酸残基突变为亮氨酸可大大加速原纤维形成过程,但会产生不同的毒性。使用一系列生物物理技术,我们证明了这些结构重复中复合甘氨酸残基的独特性。我们使用溶剂弛豫NMR光谱研究了周围水分子在确定相应的聚集途径中所起的作用。值得注意的是,由Gly33和Gly37突变诱导的构象变化导致神经元细胞系的毒性显着降低。我们的结果表明,G33 xxxG37是引起Aβ神经毒性的主要基序,因此提供了直接的结构-功能相关性。因此,靶向该基序可能是防止与阿尔茨海默氏病和其他相关疾病(例如II型糖尿病和帕金森氏病)相关的神经元细胞死亡的一种有前途的策略。
更新日期:2019-12-03
中文翻译:

β-淀粉样蛋白(21-40)肽片段的自组装和神经毒性:GxxxG母题的调节作用。
来自β-淀粉样蛋白(Aβ)肽C端区域的三个GxxxG重复基序在调节该肽的聚集动力学中起重要作用。这些甘氨酸残基突变为亮氨酸可大大加速原纤维形成过程,但会产生不同的毒性。使用一系列生物物理技术,我们证明了这些结构重复中复合甘氨酸残基的独特性。我们使用溶剂弛豫NMR光谱研究了周围水分子在确定相应的聚集途径中所起的作用。值得注意的是,由Gly33和Gly37突变诱导的构象变化导致神经元细胞系的毒性显着降低。我们的结果表明,G33 xxxG37是引起Aβ神经毒性的主要基序,因此提供了直接的结构-功能相关性。因此,靶向该基序可能是防止与阿尔茨海默氏病和其他相关疾病(例如II型糖尿病和帕金森氏病)相关的神经元细胞死亡的一种有前途的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号