当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism for hydrolysis of double six‐membered ring tetraborate anion
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-23 , DOI: 10.1002/qua.26118 Hongxia Zhou 1 , Fayan Zhu 2, 3 , Hongyan Liu 2, 3 , Wenqian Zhang 2, 3 , Yongquan Zhou 2, 3 , Chunhui Fang 2, 3
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-23 , DOI: 10.1002/qua.26118 Hongxia Zhou 1 , Fayan Zhu 2, 3 , Hongyan Liu 2, 3 , Wenqian Zhang 2, 3 , Yongquan Zhou 2, 3 , Chunhui Fang 2, 3
Affiliation
|
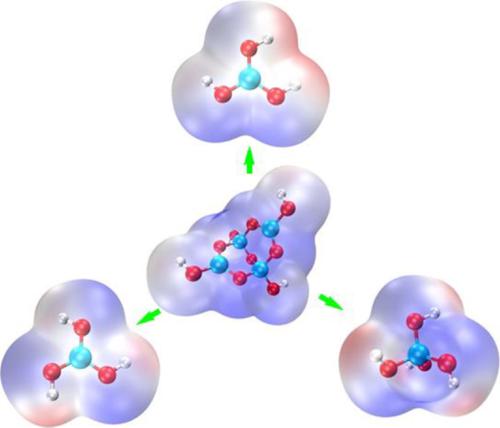
[B4O5(OH)42−] is a representative borate anion with a double six‐membered ring structure, but there is limited knowledge about the hydrolysis mechanisms of [B4O5(OH)42−]. Density functional theory‐based calculations show that the tetraborate ion undergoes three‐step hydrolysis to form [B(OH)4−] and an ring intermediate, [B3O2(OH)6−]. Other new structures, such as linear trimer, branched tetraborate, analogous linear tetraborate, are observed, but they are not stable in neutral systems and change to ring structures. [B3O2(OH)6−] hydrolyzes to [B(OH)4−] and [B(OH)3] in the last two steps. The structure of borate anion and the coordination environment of the bridge oxygen atom control the hydrolysis process. [B4O5(OH)42−] always participates in the hydrolysis reaction, even with a decrease in concentration. [B3O3(OH)4−], [B(OH)4−], and [B(OH)3] have different roles in “water‐poor” and “water‐rich” zones. Concentration and pH of solution are the key factors that affect the distribution of borate ions.
中文翻译:

双六元环四硼酸根阴离子的水解机理
[B 4 O 5(OH)4 2- ]是具有双六元环结构的代表性硼酸根阴离子,但对[B 4 O 5(OH)4 2- ]的水解机理知之甚少。密度泛函理论为基础的计算结果表明,四硼酸离子经历三步水解形成[B(OH)4 -〕和环中间体,[B 3 Ô 2(OH)6 - ]。观察到其他新结构,例如线性三聚体,支链四硼酸酯,类似的线性四硼酸酯,但它们在中性系统中不稳定,并变为环状结构。[B3 Ô 2(OH)6 - ]水解为[B(OH)4 - ]和[B(OH)3 ]中的最后两个步骤。硼酸根阴离子的结构和桥氧原子的配位环境控制着水解过程。即使浓度降低,[B 4 O 5(OH)4 2- ]也总是参与水解反应。[B 3 ø 3(OH)4 - ],[B(OH)4 - ],和[B(OH)3]在“缺水”和“缺水”区域中的作用不同。溶液的浓度和pH是影响硼酸根离子分布的关键因素。
更新日期:2020-01-23
中文翻译:

双六元环四硼酸根阴离子的水解机理
[B 4 O 5(OH)4 2- ]是具有双六元环结构的代表性硼酸根阴离子,但对[B 4 O 5(OH)4 2- ]的水解机理知之甚少。密度泛函理论为基础的计算结果表明,四硼酸离子经历三步水解形成[B(OH)4 -〕和环中间体,[B 3 Ô 2(OH)6 - ]。观察到其他新结构,例如线性三聚体,支链四硼酸酯,类似的线性四硼酸酯,但它们在中性系统中不稳定,并变为环状结构。[B3 Ô 2(OH)6 - ]水解为[B(OH)4 - ]和[B(OH)3 ]中的最后两个步骤。硼酸根阴离子的结构和桥氧原子的配位环境控制着水解过程。即使浓度降低,[B 4 O 5(OH)4 2- ]也总是参与水解反应。[B 3 ø 3(OH)4 - ],[B(OH)4 - ],和[B(OH)3]在“缺水”和“缺水”区域中的作用不同。溶液的浓度和pH是影响硼酸根离子分布的关键因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号