当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiphase Methods in Organic Electrosynthesis.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-11-25 , DOI: 10.1021/acs.accounts.9b00480 Frank Marken 1 , Jay D Wadhawan 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-11-25 , DOI: 10.1021/acs.accounts.9b00480 Frank Marken 1 , Jay D Wadhawan 2
Affiliation
|
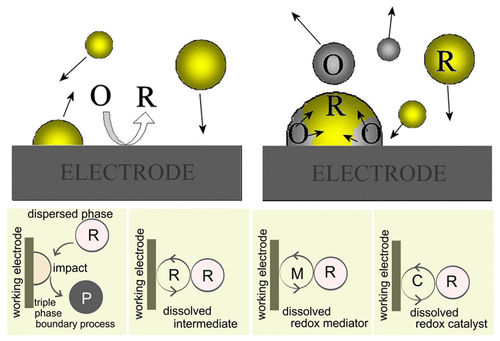
With water providing a highly favored solution environment for industrial processes (and in biological processes), it is interesting to develop water-based electrolysis processes for the synthesis and conversion of organic and biomass-based molecules. Molecules with low solubility in aqueous media can be dispersed/solubilized (i) by physical dispersion tools (e.g., milling, power ultrasound, or high-shear ultraturrax processing), (ii) in some cases by pressurization/supersaturation (e.g., for gases), (iii) by adding cosolvents or "carriers" such as chremophor EL, or (iv) by adding surfactants to generate micelles, microemulsions, and/or stabilized biphasic conditions. This Account examines and compares methodologies to bring the dispersed or multiphase system into contact with an electrode. Both the microscopic process based on individual particle impact and the overall electro-organic transformation are of interest. Distinct mechanistic cases for multiphase redox processes are considered. Most traditional electro-organic transformations are performed in homogeneous solution with reagents, products, electrolyte, and possibly mediators or redox catalysts all in the same (usually organic) solution phase. This may lead to challenges in the product separation step and in the reuse of solvents and electrolytes. When aqueous electrolyte media are used, reagents and products (or even the electrolyte) may be present as microdroplets or nanoparticles. Redox transformations then occur during interfacial "collisions" under multiphase conditions or within a reaction layer when a redox mediator is present. Benefits of this approach can be (i) the use of a highly conducting aqueous electrolyte, (ii) simple separation of products and reuse of the electrolyte, (iii) phase-transfer conditions in redox catalysis, (iv) new reaction pathways, and (v) improved sustainability. In some cases, a surface phase or phase boundary processes can lead to interesting changes in reaction pathways. Controlling the reaction zone within the multiphase redox system poses a challenge, and methods based on microchannel flow reactors have been developed to provide a higher degree of control. However, detrimental effects in microchannel systems are also observed, in particular for limited current densities (which can be very low in microchannel multiphase flow) or in the development of technical solutions for scale-up of multiphase redox transformations. This Account describes physical approaches (and reactor designs) to bring multiphase redox systems into effective contact with the electrode surface as well as cases of important electro-organic multiphase transformations. Mechanistic cases considered are "impacts" by microdroplets or particles at the electrode, effects of dissolved intermediates or redox mediators, and effects of dissolved redox catalysts. These mechanistic cases are discussed for important multiphase transformations for gaseous, liquid, and solid dispersed phases. Processes based on mesoporous membranes and hydrogen-permeable palladium membranes are discussed.
中文翻译:

有机电合成中的多相方法。
由于水为工业过程(以及生物过程)提供了非常有利的解决方案环境,因此开发用于有机和基于生物质的分子的合成和转化的水基电解过程非常有趣。在水性介质中溶解度低的分子可以(i)通过物理分散工具(例如,研磨,功率超声或高剪切Ultraturrax处理)进行分散/增溶,(ii)在某些情况下,通过加压/过饱和(例如,对于气体)进行分散/增溶。 ),(iii)通过添加助溶剂或“载体”(例如变色法EL),或(iv)通过添加表面活性剂以产生胶束,微乳液和/或稳定的双相条件。该帐户检查并比较了使分散或多相系统与电极接触的方法。基于单个粒子撞击的微观过程和整体的有机电转化都令人感兴趣。考虑了多相氧化还原过程的不同机理。大多数传统的有机电转化均在均相溶液中进行,所有试剂,产物,电解质以及可能的介体或氧化还原催化剂均在同一(通常为有机)溶液相中进行。这可能导致产品分离步骤以及溶剂和电解质的再利用方面的挑战。当使用水性电解质介质时,试剂和产物(甚至电解质)可能以微滴或纳米颗粒的形式存在。然后,在多相条件下的界面“碰撞”期间或存在氧化还原介体的情况下,在反应层内发生氧化还原转变。这种方法的好处可能是(i)使用高导电性的水性电解质;(ii)产品的简单分离和电解质的再利用;(iii)氧化还原催化中的相转移条件;(iv)新的反应途径;以及(v)改善可持续性。在某些情况下,表面相或相边界过程可能导致反应路径发生有趣的变化。在多相氧化还原系统中控制反应区提出了挑战,并且已经开发了基于微通道流动反应器的方法以提供更高程度的控制。然而,还观察到在微通道系统中的有害影响,特别是对于有限的电流密度(在微通道多相流中可能非常低)或在扩大多相氧化还原转化的技术解决方案的开发中。该帐户描述了使多相氧化还原系统与电极表面有效接触的物理方法(以及反应器设计),以及重要的电-有机多相转变的情况。所考虑的机械情况是电极上的微滴或微粒的“影响”,溶解的中间体或氧化还原介体的作用以及溶解的氧化还原催化剂的作用。讨论了这些机理案例,探讨了气态,液态和固态分散相的重要多相转化。讨论了基于介孔膜和氢可渗透钯膜的工艺。所考虑的机械情况是电极上的微滴或微粒的“影响”,溶解的中间体或氧化还原介体的作用以及溶解的氧化还原催化剂的作用。讨论了这些机理案例,探讨了气态,液态和固态分散相的重要多相转化。讨论了基于介孔膜和氢可渗透钯膜的工艺。所考虑的机械情况是电极上的微滴或微粒的“影响”,溶解的中间体或氧化还原介体的作用以及溶解的氧化还原催化剂的作用。讨论了这些机理案例,探讨了气态,液态和固态分散相的重要多相转化。讨论了基于介孔膜和氢可渗透钯膜的工艺。
更新日期:2019-11-28
中文翻译:

有机电合成中的多相方法。
由于水为工业过程(以及生物过程)提供了非常有利的解决方案环境,因此开发用于有机和基于生物质的分子的合成和转化的水基电解过程非常有趣。在水性介质中溶解度低的分子可以(i)通过物理分散工具(例如,研磨,功率超声或高剪切Ultraturrax处理)进行分散/增溶,(ii)在某些情况下,通过加压/过饱和(例如,对于气体)进行分散/增溶。 ),(iii)通过添加助溶剂或“载体”(例如变色法EL),或(iv)通过添加表面活性剂以产生胶束,微乳液和/或稳定的双相条件。该帐户检查并比较了使分散或多相系统与电极接触的方法。基于单个粒子撞击的微观过程和整体的有机电转化都令人感兴趣。考虑了多相氧化还原过程的不同机理。大多数传统的有机电转化均在均相溶液中进行,所有试剂,产物,电解质以及可能的介体或氧化还原催化剂均在同一(通常为有机)溶液相中进行。这可能导致产品分离步骤以及溶剂和电解质的再利用方面的挑战。当使用水性电解质介质时,试剂和产物(甚至电解质)可能以微滴或纳米颗粒的形式存在。然后,在多相条件下的界面“碰撞”期间或存在氧化还原介体的情况下,在反应层内发生氧化还原转变。这种方法的好处可能是(i)使用高导电性的水性电解质;(ii)产品的简单分离和电解质的再利用;(iii)氧化还原催化中的相转移条件;(iv)新的反应途径;以及(v)改善可持续性。在某些情况下,表面相或相边界过程可能导致反应路径发生有趣的变化。在多相氧化还原系统中控制反应区提出了挑战,并且已经开发了基于微通道流动反应器的方法以提供更高程度的控制。然而,还观察到在微通道系统中的有害影响,特别是对于有限的电流密度(在微通道多相流中可能非常低)或在扩大多相氧化还原转化的技术解决方案的开发中。该帐户描述了使多相氧化还原系统与电极表面有效接触的物理方法(以及反应器设计),以及重要的电-有机多相转变的情况。所考虑的机械情况是电极上的微滴或微粒的“影响”,溶解的中间体或氧化还原介体的作用以及溶解的氧化还原催化剂的作用。讨论了这些机理案例,探讨了气态,液态和固态分散相的重要多相转化。讨论了基于介孔膜和氢可渗透钯膜的工艺。所考虑的机械情况是电极上的微滴或微粒的“影响”,溶解的中间体或氧化还原介体的作用以及溶解的氧化还原催化剂的作用。讨论了这些机理案例,探讨了气态,液态和固态分散相的重要多相转化。讨论了基于介孔膜和氢可渗透钯膜的工艺。所考虑的机械情况是电极上的微滴或微粒的“影响”,溶解的中间体或氧化还原介体的作用以及溶解的氧化还原催化剂的作用。讨论了这些机理案例,探讨了气态,液态和固态分散相的重要多相转化。讨论了基于介孔膜和氢可渗透钯膜的工艺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号