当前位置:
X-MOL 学术
›
J. Proteomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A meta-analysis of affinity purification-mass spectrometry experimental systems used to identify eukaryotic and chlamydial proteins at the Chlamydia trachomatis inclusion membrane.
Journal of Proteomics ( IF 2.8 ) Pub Date : 2019-11-21 , DOI: 10.1016/j.jprot.2019.103595 Macy G Olson 1 , Scot P Ouellette 1 , Elizabeth A Rucks 1
Journal of Proteomics ( IF 2.8 ) Pub Date : 2019-11-21 , DOI: 10.1016/j.jprot.2019.103595 Macy G Olson 1 , Scot P Ouellette 1 , Elizabeth A Rucks 1
Affiliation
|
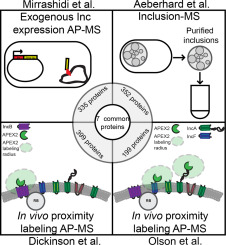
The obligate intracellular bacterial pathogen, Chlamydia trachomatis, develops within a membrane-bound vacuole termed the inclusion. Affinity purification-mass spectrometry (AP-MS) experiments to study the interactions that occur at the chlamydial inclusion membrane have been performed and, more recently, combined with advances in C. trachomatis genetics. However, each of the four AP-MS published reports used either different experimental approaches or statistical tools to identify proteins that localize at the inclusion. We critically analyzed each experimental approach and performed a meta-analysis of the reported statistically significant proteins for each study, finding that only a few eukaryotic proteins were commonly identified between all four experimental approaches. The two similarly conducted in vivo labeling studies were compared using the same statistical analysis tool, Significance Analysis of INTeractome (SAINT), which revealed a disparity in the number of significant proteins identified by the original analysis. We further examined methods to identify potential background contaminant proteins that remain after statistical analysis. Overall, this meta-analysis highlights the importance of carefully controlling and analyzing the AP-MS data so that pertinent information can be obtained from these various AP-MS experimental approaches. This study provides important guidelines and considerations for using this methodology to study intracellular pathogens residing within a membrane-bound compartment. SIGNIFICANCE: Chlamydia trachomatis, an obligate intracellular pathogen, grows within a membrane-bound vacuole termed the inclusion. The inclusion is studded with bacterial membrane proteins that likely orchestrate numerous interactions with the host cell. Although maintenance of the intracellular niche is vital, an understanding of the host-pathogen interactions that occur at the inclusion membrane is limited by the difficulty in purifying membrane protein fractions from infected host cells. The experimental procedures necessary to solubilize hydrophobic proteins fail to maintain transient protein-protein interactions. Advances in C. trachomatis genetics has allowed us and others to use various experimental approaches in combination with affinity purification mass spectrometry (AP-MS) to study the interactions that occur at the chlamydial vacuolar, or inclusion, membrane. For the first time, two groups have published AP-MS studies using the same tool, the ascorbate peroxidase proximity labeling system (APEX2), which overcomes past experimental limitations because membrane protein interactions are labeled in vivo in the context of infection. The utility of this system is highlighted by its ability to study chlamydial type III secreted inclusion membrane protein (Inc) interactions. Incs act as the mediators of host-pathogen interactions at the inclusion during C. trachomatis infection. When carefully controlled and analyzed, the data obtained can yield copious amounts of useful information. Here, we critically analyzed four previously published studies, including statistical analysis of AP-MS datasets related to Chlamydia-host interactions, to contextualize the data and to identify the best practices in interpreting these types of complex outputs.
中文翻译:

对亲和纯化质谱实验系统进行的荟萃分析,用于鉴定沙眼衣原体包膜中的真核和衣原体蛋白。
专性的细胞内细菌病原体沙眼衣原体,在被称为包涵体的膜结合液泡中发育。已经进行了亲和纯化-质谱(AP-MS)实验,以研究衣原体包膜中发生的相互作用,并且最近结合了沙眼衣原体遗传学方面的进展。但是,AP-MS发表的四个报告中的每一个都使用了不同的实验方法或统计工具来鉴定定位在包含物中的蛋白质。我们对每种实验方法进行了严格的分析,并对每项研究的报道的具有统计意义的蛋白质进行了荟萃分析,发现在这四种实验方法之间通常只能识别出少数真核蛋白质。使用相同的统计分析工具INTeractome的显着性分析(SAINT)比较了两个类似进行的体内标记研究,该分析方法揭示了通过原始分析鉴定出的重要蛋白质数量上的差异。我们进一步检查了确定统计分析后残留的潜在背景污染物蛋白质的方法。总体而言,这种荟萃分析突出了仔细控制和分析AP-MS数据的重要性,以便可以从这些各种AP-MS实验方法中获得相关信息。这项研究为使用这种方法研究膜结合区室中的细胞内病原体提供了重要的指导原则和考虑因素。重要性:沙眼衣原体,一种专性的细胞内病原体,在称为结合物的膜结合液泡中生长。该内含物散布着细菌膜蛋白,这些蛋白可能会协调与宿主细胞的许多相互作用。尽管维持细胞内的生态位至关重要,但由于难以从感染的宿主细胞中纯化膜蛋白组分,因此对包涵膜上发生的宿主-病原体相互作用的理解受到限制。溶解疏水蛋白所必需的实验程序不能维持瞬时蛋白-蛋白相互作用。沙眼衣原体遗传学的进展已使我们和其他人可以结合亲和纯化质谱(AP-MS)使用各种实验方法来研究衣原体膜或包涵体膜中发生的相互作用。首次,两组已经使用相同的工具抗坏血酸过氧化物酶邻近标记系统(APEX2)发表了AP-MS研究,该系统克服了过去的实验局限性,因为膜蛋白相互作用是在感染的情况下在体内进行标记的。该系统的实用性因其研究III型衣原体分泌性包涵体膜蛋白(Inc)相互作用的能力而突出。Incs在沙眼衣原体感染期间充当宿主-病原体相互作用的介体。当仔细地控制和分析时,获得的数据可以产生大量有用的信息。在这里,我们对以前发表的四项研究进行了批判性分析,包括与衣原体-宿主相互作用相关的AP-MS数据集的统计分析,
更新日期:2019-11-22
中文翻译:

对亲和纯化质谱实验系统进行的荟萃分析,用于鉴定沙眼衣原体包膜中的真核和衣原体蛋白。
专性的细胞内细菌病原体沙眼衣原体,在被称为包涵体的膜结合液泡中发育。已经进行了亲和纯化-质谱(AP-MS)实验,以研究衣原体包膜中发生的相互作用,并且最近结合了沙眼衣原体遗传学方面的进展。但是,AP-MS发表的四个报告中的每一个都使用了不同的实验方法或统计工具来鉴定定位在包含物中的蛋白质。我们对每种实验方法进行了严格的分析,并对每项研究的报道的具有统计意义的蛋白质进行了荟萃分析,发现在这四种实验方法之间通常只能识别出少数真核蛋白质。使用相同的统计分析工具INTeractome的显着性分析(SAINT)比较了两个类似进行的体内标记研究,该分析方法揭示了通过原始分析鉴定出的重要蛋白质数量上的差异。我们进一步检查了确定统计分析后残留的潜在背景污染物蛋白质的方法。总体而言,这种荟萃分析突出了仔细控制和分析AP-MS数据的重要性,以便可以从这些各种AP-MS实验方法中获得相关信息。这项研究为使用这种方法研究膜结合区室中的细胞内病原体提供了重要的指导原则和考虑因素。重要性:沙眼衣原体,一种专性的细胞内病原体,在称为结合物的膜结合液泡中生长。该内含物散布着细菌膜蛋白,这些蛋白可能会协调与宿主细胞的许多相互作用。尽管维持细胞内的生态位至关重要,但由于难以从感染的宿主细胞中纯化膜蛋白组分,因此对包涵膜上发生的宿主-病原体相互作用的理解受到限制。溶解疏水蛋白所必需的实验程序不能维持瞬时蛋白-蛋白相互作用。沙眼衣原体遗传学的进展已使我们和其他人可以结合亲和纯化质谱(AP-MS)使用各种实验方法来研究衣原体膜或包涵体膜中发生的相互作用。首次,两组已经使用相同的工具抗坏血酸过氧化物酶邻近标记系统(APEX2)发表了AP-MS研究,该系统克服了过去的实验局限性,因为膜蛋白相互作用是在感染的情况下在体内进行标记的。该系统的实用性因其研究III型衣原体分泌性包涵体膜蛋白(Inc)相互作用的能力而突出。Incs在沙眼衣原体感染期间充当宿主-病原体相互作用的介体。当仔细地控制和分析时,获得的数据可以产生大量有用的信息。在这里,我们对以前发表的四项研究进行了批判性分析,包括与衣原体-宿主相互作用相关的AP-MS数据集的统计分析,











































 京公网安备 11010802027423号
京公网安备 11010802027423号