当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic mechanisms of oxygen-containing groups over vanadium active sites in an Al-MCM-41 framework for production of 2,5-diformylfuran from 5-hydroxymethylfurfural
Catalysis Science & Technology ( IF 5 ) Pub Date : 2019-11-22 , DOI: 10.1039/c9cy02130b Li-Juan Liu 1, 2, 3, 4 , Zhao-Meng Wang 1, 2, 3, 4 , Ya-Jing Lyu 2, 3, 4, 5, 6 , Jin-Feng Zhang 1, 2, 3, 4 , Zhou Huang 1, 2, 3, 4 , Ting Qi 1, 2, 3, 4 , Zhen-Bing Si 1, 2, 3, 4 , Hua-Qing Yang 1, 2, 3, 4 , Chang-Wei Hu 2, 3, 4, 5, 6
Catalysis Science & Technology ( IF 5 ) Pub Date : 2019-11-22 , DOI: 10.1039/c9cy02130b Li-Juan Liu 1, 2, 3, 4 , Zhao-Meng Wang 1, 2, 3, 4 , Ya-Jing Lyu 2, 3, 4, 5, 6 , Jin-Feng Zhang 1, 2, 3, 4 , Zhou Huang 1, 2, 3, 4 , Ting Qi 1, 2, 3, 4 , Zhen-Bing Si 1, 2, 3, 4 , Hua-Qing Yang 1, 2, 3, 4 , Chang-Wei Hu 2, 3, 4, 5, 6
Affiliation
|
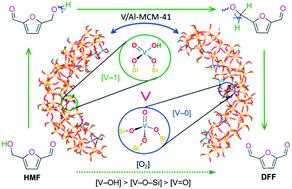
V-containing catalysts exhibit good catalytic performance toward the selective oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF). Here, we report our study on the catalytic mechanism of –(SiO)3V(O) ([V-0]) and –(SiO)2V(O)(OH) ([V-1]) on a V-doped Al-MCM-41 pore model (V/Al-MCM-41) for the aerobic oxidation of HMF to DFF. For the two active sites, there are three types of oxygen-containing functional groups, which are hydroxyl-oxygen ([V–OH]), lattice-oxygen ([V–O–Si]), and terminal-oxygen ([V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]). We show that the catalytic cycle involves two HMF molecules, and there are mainly two activation steps, i.e., both O–H and C–H bond cleavages of HMF, and the rate-determining step is associated with the C–H bond cleavage of the first HMF molecule. We illustrate the efficiency of the catalytic contribution as [V–OH] > [V–O–Si] > [V
O]). We show that the catalytic cycle involves two HMF molecules, and there are mainly two activation steps, i.e., both O–H and C–H bond cleavages of HMF, and the rate-determining step is associated with the C–H bond cleavage of the first HMF molecule. We illustrate the efficiency of the catalytic contribution as [V–OH] > [V–O–Si] > [V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O], and the [V-1] active site with a hydroxyl group displays higher catalytic activity than the [V-0] active site without a hydroxyl group. The present study not only brings an in-depth understanding of the activation of both O–H and C–H bonds which has been proposed based on experimental results for biomass molecules, but also makes one step forward toward the mechanism-guided design and synthesis of efficient, environmentally-friendly, and low temperature recyclable heterogeneous catalysts.
O], and the [V-1] active site with a hydroxyl group displays higher catalytic activity than the [V-0] active site without a hydroxyl group. The present study not only brings an in-depth understanding of the activation of both O–H and C–H bonds which has been proposed based on experimental results for biomass molecules, but also makes one step forward toward the mechanism-guided design and synthesis of efficient, environmentally-friendly, and low temperature recyclable heterogeneous catalysts.
中文翻译:

Al-MCM-41骨架中钒活性位上含氧基团的催化机理,用于由5-羟甲基糠醛生产2,5-二甲酰呋喃
含V的催化剂对5-羟甲基糠醛(HMF)选择性氧化为2,5-二甲酰呋喃(DFF)表现出良好的催化性能。在这里,我们报告了我们对–(SiO)3 V(O)([V-0])和–(SiO)2 V(O)(OH)([V-1])在V上的催化机理的研究。掺杂的Al-MCM-41孔模型(V / Al-MCM-41),用于HMF有氧氧化为DFF。对于这两个活性位,存在三种类型的含氧官能团,分别是羟基氧([V–OH]),晶格氧([V–O–Si])和末端氧([V![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O])。我们表明催化循环涉及两个HMF分子,并且主要有两个激活步骤,即,HMF的O–H和C–H键均断裂,并且速率确定步骤与第一个HMF分子的C–H键断裂相关。我们说明作为催化贡献的效率[V-OH]> [V-O-的Si]> [V
O])。我们表明催化循环涉及两个HMF分子,并且主要有两个激活步骤,即,HMF的O–H和C–H键均断裂,并且速率确定步骤与第一个HMF分子的C–H键断裂相关。我们说明作为催化贡献的效率[V-OH]> [V-O-的Si]> [V ![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O]和[V-1]的活性位点与羟基显示比更高的催化活性[V- 0]没有羟基的活性位。本研究不仅对基于生物质分子的实验结果提出的O–H和C–H键的激活进行了深入了解,而且还朝着机理指导的设计和合成迈出了一步高效,环保和低温可循环使用的多相催化剂。
O]和[V-1]的活性位点与羟基显示比更高的催化活性[V- 0]没有羟基的活性位。本研究不仅对基于生物质分子的实验结果提出的O–H和C–H键的激活进行了深入了解,而且还朝着机理指导的设计和合成迈出了一步高效,环保和低温可循环使用的多相催化剂。
更新日期:2019-11-22
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]). We show that the catalytic cycle involves two HMF molecules, and there are mainly two activation steps, i.e., both O–H and C–H bond cleavages of HMF, and the rate-determining step is associated with the C–H bond cleavage of the first HMF molecule. We illustrate the efficiency of the catalytic contribution as [V–OH] > [V–O–Si] > [V
O]). We show that the catalytic cycle involves two HMF molecules, and there are mainly two activation steps, i.e., both O–H and C–H bond cleavages of HMF, and the rate-determining step is associated with the C–H bond cleavage of the first HMF molecule. We illustrate the efficiency of the catalytic contribution as [V–OH] > [V–O–Si] > [V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O], and the [V-1] active site with a hydroxyl group displays higher catalytic activity than the [V-0] active site without a hydroxyl group. The present study not only brings an in-depth understanding of the activation of both O–H and C–H bonds which has been proposed based on experimental results for biomass molecules, but also makes one step forward toward the mechanism-guided design and synthesis of efficient, environmentally-friendly, and low temperature recyclable heterogeneous catalysts.
O], and the [V-1] active site with a hydroxyl group displays higher catalytic activity than the [V-0] active site without a hydroxyl group. The present study not only brings an in-depth understanding of the activation of both O–H and C–H bonds which has been proposed based on experimental results for biomass molecules, but also makes one step forward toward the mechanism-guided design and synthesis of efficient, environmentally-friendly, and low temperature recyclable heterogeneous catalysts.
中文翻译:

Al-MCM-41骨架中钒活性位上含氧基团的催化机理,用于由5-羟甲基糠醛生产2,5-二甲酰呋喃
含V的催化剂对5-羟甲基糠醛(HMF)选择性氧化为2,5-二甲酰呋喃(DFF)表现出良好的催化性能。在这里,我们报告了我们对–(SiO)3 V(O)([V-0])和–(SiO)2 V(O)(OH)([V-1])在V上的催化机理的研究。掺杂的Al-MCM-41孔模型(V / Al-MCM-41),用于HMF有氧氧化为DFF。对于这两个活性位,存在三种类型的含氧官能团,分别是羟基氧([V–OH]),晶格氧([V–O–Si])和末端氧([V
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O])。我们表明催化循环涉及两个HMF分子,并且主要有两个激活步骤,即,HMF的O–H和C–H键均断裂,并且速率确定步骤与第一个HMF分子的C–H键断裂相关。我们说明作为催化贡献的效率[V-OH]> [V-O-的Si]> [V
O])。我们表明催化循环涉及两个HMF分子,并且主要有两个激活步骤,即,HMF的O–H和C–H键均断裂,并且速率确定步骤与第一个HMF分子的C–H键断裂相关。我们说明作为催化贡献的效率[V-OH]> [V-O-的Si]> [V ![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O]和[V-1]的活性位点与羟基显示比更高的催化活性[V- 0]没有羟基的活性位。本研究不仅对基于生物质分子的实验结果提出的O–H和C–H键的激活进行了深入了解,而且还朝着机理指导的设计和合成迈出了一步高效,环保和低温可循环使用的多相催化剂。
O]和[V-1]的活性位点与羟基显示比更高的催化活性[V- 0]没有羟基的活性位。本研究不仅对基于生物质分子的实验结果提出的O–H和C–H键的激活进行了深入了解,而且还朝着机理指导的设计和合成迈出了一步高效,环保和低温可循环使用的多相催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号