当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Insight into the Mechanism of Inhibitor Resistance in CTX-M-199, a CTX-M-64 Variant Carrying the S130T Substitution.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2019-11-21 , DOI: 10.1021/acsinfecdis.9b00345 Qipeng Cheng 1 , Chen Xu 1 , Jiachang Chai 2 , Rong Zhang 2 , Edward Wai Chi Chan 1 , Sheng Chen 3
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2019-11-21 , DOI: 10.1021/acsinfecdis.9b00345 Qipeng Cheng 1 , Chen Xu 1 , Jiachang Chai 2 , Rong Zhang 2 , Edward Wai Chi Chan 1 , Sheng Chen 3
Affiliation
|
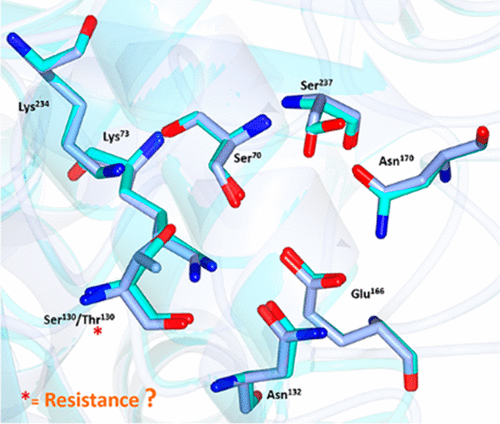
The smart design of β-lactamase inhibitors allowed us to combat extended-spectrum β-lactamase (ESBL)-producing organisms for many years without developing resistance to these inhibitors. However, novel resistant variants have emerged recently, and notable examples are the CTX-M-190 and CTX-M-199 variants, which carried a S130T amino acid substitution and exhibited resistance to inhibitors such as sulbactam and tazobactam. Using mass spectrometric and crystallographic approaches, this study depicted the mechanisms of inhibitor resistance. Our data showed that CTX-M-64 (S130T) did not cause any conformational change or exert any effect on its ability to hydrolyze β-lactam substrates. However, binding of sulbactam, but not clavulanic acid, to the active site of CTX-M-64 (S130T) led to the conformational changes in such active site, which comprised the key residues involved in substrate catalysis, namely, Thr130, Lys73, Lys234, Asn104, and Asn132. This conformational change weakened the binding of the sulbactam trans-enamine intermediate (TSL) to the active site and rendered the formation of the inhibitor-enzyme complex, which features a covalent acrylic acid (AKR)-T130 bond, inefficient, thereby resulting in inhibitor resistance in CTX-M-64 (S130T). Understanding the mechanisms of inhibitor resistance provided structural insight for the future development of new inhibitors against inhibitor-resistant β-lactamases.
中文翻译:

对CTX-M-199(带有S130T替代物的CTX-M-64变体)中的抗药性机理的结构性见解。
β-内酰胺酶抑制剂的精巧设计使我们能够与生产广谱β-内酰胺酶(ESBL)的生物抗争多年,而不会产生对这些抑制剂的抗性。然而,最近出现了新的抗性变体,并且值得注意的例子是CTX-M-190和CTX-M-199变体,其带有S130T氨基酸取代并表现出对诸如舒巴坦和他唑巴坦的抑制剂的抗性。使用质谱和晶体学方法,该研究描述了抑制剂抗性的机制。我们的数据表明CTX-M-64(S130T)不会引起任何构象变化或对其水解β-内酰胺底物的能力产生任何影响。但是,舒巴坦(而非克拉维酸)与CTX-M-64(S130T)的活性位点结合会导致这种活性位点发生构象变化,其包含参与底物催化的关键残基,即Thr130,Lys73,Lys234,Asn104和Asn132。这种构象变化削弱了舒巴坦反式烯胺中间体(TSL)与活性位点的结合,并形成了抑制剂-酶复合物,该复合物具有共价丙烯酸(AKR)-T130键,效率低下,从而导致了抑制剂的产生。 CTX-M-64(S130T)中的电阻。了解抑制剂抗性的机制为抗抑制剂β-内酰胺酶的新型抑制剂的未来开发提供了结构上的见识。这种构象变化削弱了舒巴坦反式烯胺中间体(TSL)与活性位点的结合,并形成了抑制剂-酶复合物,该复合物具有共价丙烯酸(AKR)-T130键,效率低下,从而导致了抑制剂的产生。 CTX-M-64(S130T)中的电阻。了解抑制剂抗性的机制为抗抑制剂β-内酰胺酶的新型抑制剂的未来开发提供了结构上的见识。这种构象变化削弱了舒巴坦反式烯胺中间体(TSL)与活性位点的结合,并形成了抑制剂-酶复合物,该复合物具有共价丙烯酸(AKR)-T130键,效率低下,从而导致了抑制剂的产生。 CTX-M-64(S130T)中的电阻。了解抑制剂抗性的机制为抗抑制剂β-内酰胺酶的新型抑制剂的未来开发提供了结构上的见识。
更新日期:2019-11-11
中文翻译:

对CTX-M-199(带有S130T替代物的CTX-M-64变体)中的抗药性机理的结构性见解。
β-内酰胺酶抑制剂的精巧设计使我们能够与生产广谱β-内酰胺酶(ESBL)的生物抗争多年,而不会产生对这些抑制剂的抗性。然而,最近出现了新的抗性变体,并且值得注意的例子是CTX-M-190和CTX-M-199变体,其带有S130T氨基酸取代并表现出对诸如舒巴坦和他唑巴坦的抑制剂的抗性。使用质谱和晶体学方法,该研究描述了抑制剂抗性的机制。我们的数据表明CTX-M-64(S130T)不会引起任何构象变化或对其水解β-内酰胺底物的能力产生任何影响。但是,舒巴坦(而非克拉维酸)与CTX-M-64(S130T)的活性位点结合会导致这种活性位点发生构象变化,其包含参与底物催化的关键残基,即Thr130,Lys73,Lys234,Asn104和Asn132。这种构象变化削弱了舒巴坦反式烯胺中间体(TSL)与活性位点的结合,并形成了抑制剂-酶复合物,该复合物具有共价丙烯酸(AKR)-T130键,效率低下,从而导致了抑制剂的产生。 CTX-M-64(S130T)中的电阻。了解抑制剂抗性的机制为抗抑制剂β-内酰胺酶的新型抑制剂的未来开发提供了结构上的见识。这种构象变化削弱了舒巴坦反式烯胺中间体(TSL)与活性位点的结合,并形成了抑制剂-酶复合物,该复合物具有共价丙烯酸(AKR)-T130键,效率低下,从而导致了抑制剂的产生。 CTX-M-64(S130T)中的电阻。了解抑制剂抗性的机制为抗抑制剂β-内酰胺酶的新型抑制剂的未来开发提供了结构上的见识。这种构象变化削弱了舒巴坦反式烯胺中间体(TSL)与活性位点的结合,并形成了抑制剂-酶复合物,该复合物具有共价丙烯酸(AKR)-T130键,效率低下,从而导致了抑制剂的产生。 CTX-M-64(S130T)中的电阻。了解抑制剂抗性的机制为抗抑制剂β-内酰胺酶的新型抑制剂的未来开发提供了结构上的见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号