当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Luminescent Europium Peptide Conjugates: Comparative Analysis Using Maleimide and para-Nitropyridyl Linkages for Organelle Staining.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2019-12-31 , DOI: 10.1021/acs.bioconjchem.9b00735 Matthieu Starck 1 , Jack D Fradgley 1 , Stefania Di Vita 1 , Jackie A Mosely 1 , Robert Pal 1 , David Parker 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2019-12-31 , DOI: 10.1021/acs.bioconjchem.9b00735 Matthieu Starck 1 , Jack D Fradgley 1 , Stefania Di Vita 1 , Jackie A Mosely 1 , Robert Pal 1 , David Parker 1
Affiliation
|
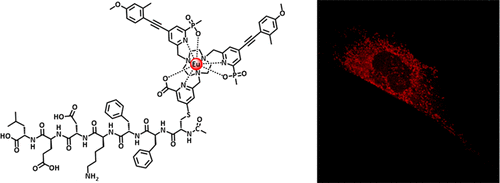
The syntheses and photophysical behavior of nine strongly luminescent nonadentate Eu(III) complexes are reported. Each complex is based on N-functionalized 1,4,7-triazacyclononane, and linkage to other groups or targeting vectors can occur either via amide bond formation to a coordinated pyridine p-aminopropyl group or via a nucleophilic substitution reaction involving thiol attack on a metal coordinated p-nitropyridyl moiety. Evidence is presented in favor of the latter conjugation strategy, as parallel work with maleimide conjugates was complicated or compromised by the propensity to undergo post-conjugation thiol exchange or succinimide ring hydrolysis reactions. Confocal microscopy and spectral imaging studies revealed that the peptide conjugate of AcCFFKDEL was found to localize selectively in the endoplasmic reticulum of mouse fibroblast cells, whereas the related maleimide conjugate was only observed in cellular lysosomes.
中文翻译:

靶向发光的P肽共轭物:使用马来酰亚胺和对硝基苯并吡啶键进行细胞器染色的比较分析。
报道了九种强发光非齿Eu(III)配合物的合成和光物理行为。每个复合物均基于N-官能化的1,4,7-三氮杂环壬烷,与其他基团或靶向载体的连接可以通过与配位吡啶对氨基丙基基团形成酰胺键,也可以通过涉及巯基攻击巯基的亲核取代反应发生。金属配位的对硝基吡啶基部分。提出了支持后者结合策略的证据,因为与马来酰亚胺结合物的平行工作因经历结合后硫醇交换或琥珀酰亚胺环水解反应的倾向而变得复杂或受到损害。
更新日期:2020-01-01
中文翻译:

靶向发光的P肽共轭物:使用马来酰亚胺和对硝基苯并吡啶键进行细胞器染色的比较分析。
报道了九种强发光非齿Eu(III)配合物的合成和光物理行为。每个复合物均基于N-官能化的1,4,7-三氮杂环壬烷,与其他基团或靶向载体的连接可以通过与配位吡啶对氨基丙基基团形成酰胺键,也可以通过涉及巯基攻击巯基的亲核取代反应发生。金属配位的对硝基吡啶基部分。提出了支持后者结合策略的证据,因为与马来酰亚胺结合物的平行工作因经历结合后硫醇交换或琥珀酰亚胺环水解反应的倾向而变得复杂或受到损害。











































 京公网安备 11010802027423号
京公网安备 11010802027423号