当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of Catalysis and Inhibition of HDAC6 CD1, the Enigmatic Catalytic Domain of Histone Deacetylase 6.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-02 , DOI: 10.1021/acs.biochem.9b00934 Jeremy D Osko 1 , David W Christianson 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-02 , DOI: 10.1021/acs.biochem.9b00934 Jeremy D Osko 1 , David W Christianson 1
Affiliation
|
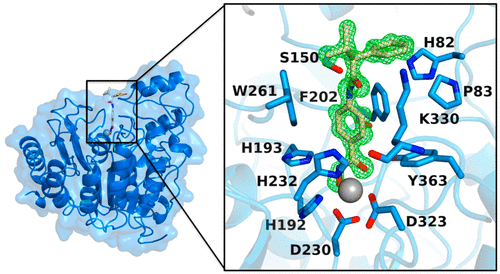
Histone deacetylase 6 (HDAC6) is emerging as a target for inhibition in therapeutic strategies aimed at treating cancer, neurodegenerative disease, and other disorders. Among the metal-dependent HDAC isozymes, HDAC6 is unique in that it contains two catalytic domains, CD1 and CD2. CD2 is a tubulin deacetylase and a tau deacetylase, and the development of HDAC6-selective inhibitors has focused exclusively on this domain. In contrast, there is a dearth of structural and functional information regarding CD1, which exhibits much narrower substrate specificity in comparison with CD2. As the first step in addressing the CD1 information gap, we now present X-ray crystal structures of seven inhibitor complexes with wild-type, Y363F, and K330L HDAC6 CD1. These structures broaden our understanding of molecular features that are important for catalysis and inhibitor binding. The active site of HDAC6 CD1 is wider than that of CD2, which is unexpected in view of the narrow substrate specificity of CD1. Amino acid substitutions between HDAC6 CD1 and CD2, as well as conformational differences in conserved residues, define striking differences in active site contours. Catalytic activity measurements with HDAC6 CD1 confirm the preference for peptide substrates containing C-terminal acetyllysine residues. However, these measurements also show that CD1 exhibits weak activity for peptide substrates bearing certain small amino acids on the carboxyl side of the scissile acetyllysine residue. Taken together, these results establish a foundation for understanding the structural basis of HDAC6 CD1 catalysis and inhibition, pointing to possible avenues for the development of HDAC6 CD1-selective inhibitors.
中文翻译:

HDAC6 CD1(组蛋白脱乙酰酶 6 的神秘催化结构域)催化和抑制的结构基础。
组蛋白脱乙酰酶 6 (HDAC6) 正在成为癌症、神经退行性疾病和其他疾病治疗策略中的抑制靶点。在金属依赖性 HDAC 同工酶中,HDAC6 的独特之处在于它包含两个催化结构域:CD1 和 CD2。 CD2 是一种微管蛋白脱乙酰酶和 tau 脱乙酰酶,HDAC6 选择性抑制剂的开发专门集中在该域上。相比之下,CD1 的结构和功能信息缺乏,与 CD2 相比,CD1 的底物特异性要窄得多。作为解决 CD1 信息缺口的第一步,我们现在展示了七种抑制剂与野生型、Y363F 和 K330L HDAC6 CD1 复合物的 X 射线晶体结构。这些结构拓宽了我们对对于催化和抑制剂结合很重要的分子特征的理解。 HDAC6 CD1 的活性位点比 CD2 的活性位点更宽,鉴于 CD1 的底物特异性较窄,这是出乎意料的。 HDAC6 CD1 和 CD2 之间的氨基酸替换以及保守残基的构象差异定义了活性位点轮廓的显着差异。 HDAC6 CD1 的催化活性测量证实了对含有 C 末端乙酰赖氨酸残基的肽底物的偏好。然而,这些测量还表明,CD1 对在可剪切的乙酰赖氨酸残基的羧基侧带有某些小氨基酸的肽底物表现出弱活性。总而言之,这些结果为理解 HDAC6 CD1 催化和抑制的结构基础奠定了基础,为开发 HDAC6 CD1 选择性抑制剂提供了可能的途径。
更新日期:2019-12-03
中文翻译:

HDAC6 CD1(组蛋白脱乙酰酶 6 的神秘催化结构域)催化和抑制的结构基础。
组蛋白脱乙酰酶 6 (HDAC6) 正在成为癌症、神经退行性疾病和其他疾病治疗策略中的抑制靶点。在金属依赖性 HDAC 同工酶中,HDAC6 的独特之处在于它包含两个催化结构域:CD1 和 CD2。 CD2 是一种微管蛋白脱乙酰酶和 tau 脱乙酰酶,HDAC6 选择性抑制剂的开发专门集中在该域上。相比之下,CD1 的结构和功能信息缺乏,与 CD2 相比,CD1 的底物特异性要窄得多。作为解决 CD1 信息缺口的第一步,我们现在展示了七种抑制剂与野生型、Y363F 和 K330L HDAC6 CD1 复合物的 X 射线晶体结构。这些结构拓宽了我们对对于催化和抑制剂结合很重要的分子特征的理解。 HDAC6 CD1 的活性位点比 CD2 的活性位点更宽,鉴于 CD1 的底物特异性较窄,这是出乎意料的。 HDAC6 CD1 和 CD2 之间的氨基酸替换以及保守残基的构象差异定义了活性位点轮廓的显着差异。 HDAC6 CD1 的催化活性测量证实了对含有 C 末端乙酰赖氨酸残基的肽底物的偏好。然而,这些测量还表明,CD1 对在可剪切的乙酰赖氨酸残基的羧基侧带有某些小氨基酸的肽底物表现出弱活性。总而言之,这些结果为理解 HDAC6 CD1 催化和抑制的结构基础奠定了基础,为开发 HDAC6 CD1 选择性抑制剂提供了可能的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号