当前位置:
X-MOL 学术
›
Nat. Rev. Mol. Cell Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The molecular principles governing the activity and functional diversity of AAA+ proteins.
Nature Reviews Molecular Cell Biology ( IF 81.3 ) Pub Date : 2019-11-21 , DOI: 10.1038/s41580-019-0183-6 Cristina Puchades 1 , Colby R Sandate 1 , Gabriel C Lander 1
Nature Reviews Molecular Cell Biology ( IF 81.3 ) Pub Date : 2019-11-21 , DOI: 10.1038/s41580-019-0183-6 Cristina Puchades 1 , Colby R Sandate 1 , Gabriel C Lander 1
Affiliation
|
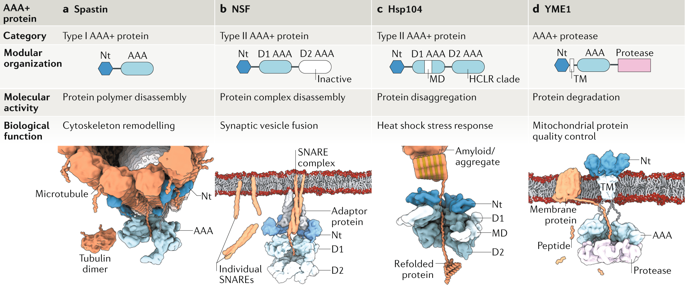
ATPases associated with diverse cellular activities (AAA+ proteins) are macromolecular machines that convert the chemical energy contained in ATP molecules into powerful mechanical forces to remodel a vast array of cellular substrates, including protein aggregates, macromolecular complexes and polymers. AAA+ proteins have key functionalities encompassing unfolding and disassembly of such substrates in different subcellular localizations and, hence, power a plethora of fundamental cellular processes, including protein quality control, cytoskeleton remodelling and membrane dynamics. Over the past 35 years, many of the key elements required for AAA+ activity have been identified through genetic, biochemical and structural analyses. However, how ATP powers substrate remodelling and whether a shared mechanism underlies the functional diversity of the AAA+ superfamily were uncertain. Advances in cryo-electron microscopy have enabled high-resolution structure determination of AAA+ proteins trapped in the act of processing substrates, revealing a conserved core mechanism of action. It has also become apparent that this common mechanistic principle is structurally adjusted to carry out a diverse array of biological functions. Here, we review how substrate-bound structures of AAA+ proteins have expanded our understanding of ATP-driven protein remodelling.
中文翻译:

控制 AAA+ 蛋白活性和功能多样性的分子原理。
与多种细胞活动相关的 ATP 酶(AAA+ 蛋白质)是一种大分子机器,可将 ATP 分子中包含的化学能转化为强大的机械力,从而重塑大量细胞底物,包括蛋白质聚集体、大分子复合物和聚合物。AAA+ 蛋白质具有关键功能,包括这些底物在不同亚细胞定位中的展开和分解,因此,为大量的基本细胞过程提供动力,包括蛋白质质量控制、细胞骨架重塑和膜动力学。在过去的 35 年中,许多 AAA+ 活性所需的关键元素已通过遗传、生化和结构分析确定。然而,ATP 如何为底物重塑提供动力以及是否存在共同机制是 AAA+ 超家族功能多样性的基础尚不确定。低温电子显微镜技术的进步已经能够对在处理底物的行为中捕获的 AAA+ 蛋白质进行高分辨率结构测定,揭示了一种保守的核心作用机制。很明显,这种常见的机械原理在结构上进行了调整,以执行各种生物功能。在这里,我们回顾了 AAA+ 蛋白质的底物结合结构如何扩大了我们对 ATP 驱动的蛋白质重塑的理解。很明显,这种常见的机械原理在结构上进行了调整,以执行各种生物功能。在这里,我们回顾了 AAA+ 蛋白质的底物结合结构如何扩大了我们对 ATP 驱动的蛋白质重塑的理解。很明显,这种常见的机械原理在结构上进行了调整,以执行各种生物功能。在这里,我们回顾了 AAA+ 蛋白质的底物结合结构如何扩大了我们对 ATP 驱动的蛋白质重塑的理解。
更新日期:2019-11-22
中文翻译:

控制 AAA+ 蛋白活性和功能多样性的分子原理。
与多种细胞活动相关的 ATP 酶(AAA+ 蛋白质)是一种大分子机器,可将 ATP 分子中包含的化学能转化为强大的机械力,从而重塑大量细胞底物,包括蛋白质聚集体、大分子复合物和聚合物。AAA+ 蛋白质具有关键功能,包括这些底物在不同亚细胞定位中的展开和分解,因此,为大量的基本细胞过程提供动力,包括蛋白质质量控制、细胞骨架重塑和膜动力学。在过去的 35 年中,许多 AAA+ 活性所需的关键元素已通过遗传、生化和结构分析确定。然而,ATP 如何为底物重塑提供动力以及是否存在共同机制是 AAA+ 超家族功能多样性的基础尚不确定。低温电子显微镜技术的进步已经能够对在处理底物的行为中捕获的 AAA+ 蛋白质进行高分辨率结构测定,揭示了一种保守的核心作用机制。很明显,这种常见的机械原理在结构上进行了调整,以执行各种生物功能。在这里,我们回顾了 AAA+ 蛋白质的底物结合结构如何扩大了我们对 ATP 驱动的蛋白质重塑的理解。很明显,这种常见的机械原理在结构上进行了调整,以执行各种生物功能。在这里,我们回顾了 AAA+ 蛋白质的底物结合结构如何扩大了我们对 ATP 驱动的蛋白质重塑的理解。很明显,这种常见的机械原理在结构上进行了调整,以执行各种生物功能。在这里,我们回顾了 AAA+ 蛋白质的底物结合结构如何扩大了我们对 ATP 驱动的蛋白质重塑的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号