当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed synthesis and nucleotide pyrophosphatase inhibition of benzo[4,5]furo[3,2-b]indoles
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2019-11-22 , DOI: 10.3762/bjoc.15.276 Hoang Huy Do , Saif Ullah , Alexander Villinger , Joanna Lecka , Jean Sévigny , Peter Ehlers , Jamshed Iqbal , Peter Langer
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2019-11-22 , DOI: 10.3762/bjoc.15.276 Hoang Huy Do , Saif Ullah , Alexander Villinger , Joanna Lecka , Jean Sévigny , Peter Ehlers , Jamshed Iqbal , Peter Langer
|
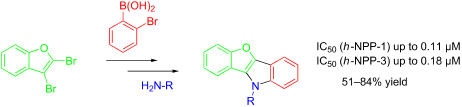
A two-step palladium-catalyzed procedure based on Suzuki–Miyaura cross coupling, followed by a double Buchwald–Hartwig reaction, allows for the synthesis of pharmaceutically relevant benzo[4,5]furo[3,2-b]indoles in moderate to very good yield. The synthesized compounds have been analyzed with regard to their inhibitory activity (IC50) of nucleotide pyrophosphatases h-NPP1 and h-NPP3. The activity lies in the nanomolar range. The results were rationalized based on docking studies.
中文翻译:

钯催化苯并[4,5]呋喃[3,2-b]吲哚的合成及其核苷酸焦磷酸酶抑制作用
根据Suzuki-Miyaura交叉偶联的两步钯催化过程,接着是双布赫瓦尔德-哈特维希反应,允许药物相关苯并[4,5]呋喃并[3,2的合成-b〕吲哚在中度至良品率很高。已对合成化合物的核苷酸焦磷酸酶h -NPP1和h -NPP3的抑制活性(IC 50)进行了分析。活性在纳摩尔范围内。根据对接研究对结果进行了合理化。
更新日期:2019-11-22
中文翻译:

钯催化苯并[4,5]呋喃[3,2-b]吲哚的合成及其核苷酸焦磷酸酶抑制作用
根据Suzuki-Miyaura交叉偶联的两步钯催化过程,接着是双布赫瓦尔德-哈特维希反应,允许药物相关苯并[4,5]呋喃并[3,2的合成-b〕吲哚在中度至良品率很高。已对合成化合物的核苷酸焦磷酸酶h -NPP1和h -NPP3的抑制活性(IC 50)进行了分析。活性在纳摩尔范围内。根据对接研究对结果进行了合理化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号