Molecular Catalysis ( IF 3.9 ) Pub Date : 2019-11-22 , DOI: 10.1016/j.mcat.2019.110718 Xi Zhou , Cuihua Zhao , Baishi li , Jianhua Chen , Liwei Wang
|
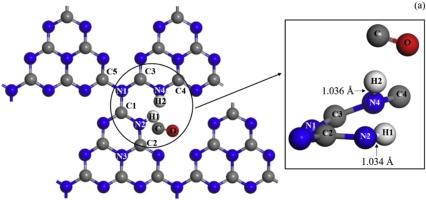
The interaction between HCHO molecule and tri-s-triazine g-C3N4 surface was studied by using DFT method. The vacancy site adsorption of HCHO molecule on g-C3N4 surface is the most stable, and calculated adsorption energy is -0.213 eV. HCHO is dissociated after adsorption with the cleavage of CH bonds (C
H1 and C
H2) and forming of N
H bonds (N2
H1 and N4
H2). The electrons transfer from HCHO to g-C3N4 surface in the course of the interaction between HCHO and g-C3N4 surface. The electrons donated by HCHO molecule are mainly from oxygen atom and hydrogen atoms. However, the electrons do not stay in nitrogen atoms (N2 and N4) bonded with hydrogen atoms (H1 and H2), while may transfer to carbon atoms by nitrogen atoms, that is, the electrons from HCHO molecule may transfer first to neighbor nitrogen atoms of g-C3N4 surface, then transfer to carbon atoms bonded with nitrogen atoms.
中文翻译:

DFT研究HCHO分子与tri-s-triazine gC 3 N 4表面之间的相互作用
利用DFT方法研究了HCHO分子与tri-s-triazine gC 3 N 4表面的相互作用。HCHO分子在gC 3 N 4表面的空位吸附最稳定,计算出的吸附能为-0.213 eV。HCHO在吸附后会裂解C H键(C
H1和C
H2)并形成N
H键(N2
H1和N4
H2)而解离。电子在HCHO和gC 3 N 4相互作用的过程中从HCHO转移到gC 3 N 4表面表面。HCHO分子提供的电子主要来自氧原子和氢原子。但是,电子不会停留在与氢原子(H1和H2)键合的氮原子(N2和N4)中,而可能会被氮原子转移到碳原子上,也就是说,来自HCHO分子的电子可能首先转移到相邻的氮原子上gC 3 N 4表面,然后转移到与氮原子键合的碳原子上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号