当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental measurement and thermodynamic modeling of Lansoprazole solubility in supercritical carbon dioxide: Application of SAFT-VR EoS
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.fluid.2019.112422 Gholamhossein Sodeifian , Seyed Ali Sajadian , Reza Derakhsheshpour
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.fluid.2019.112422 Gholamhossein Sodeifian , Seyed Ali Sajadian , Reza Derakhsheshpour
|
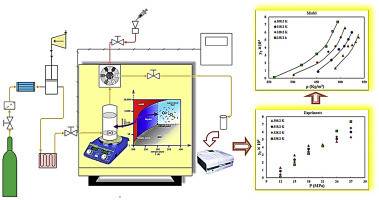
Abstract Solubility measurement of a solid solute in supercritical fluid (SCF) is considered as a primary step in micro- and nano-sized particles production through SCF technology. In this work, for the first time, solubility of Lansoprazole (LPZ) in supercritical carbon dioxide (SC–CO2) was determined at pressures and temperatures ranging within 12–27 MPa and 308.2–338.2 K, respectively. Under the applied conditions, mole fractions were obtained in the range of 1.15 × 10−5 to 7.36 × 10−4. For correlating the drug solubility, six semi-empirical models and two equations of state (EoSs), i.e., Peng–Robinson (PR) as a cubic EoS and SAFT-VR as a non-cubic EoSs were applied. Results showed the highest accuracy for “Reddy and Garlapati” model among semi-empirical models with AARDz of 7.36%. Furthermore, SAFT-VR EoS with AARD of 6.65 proved a very good priority than PR EoS and displayed the highest precision compared to all empirical models. This could be a promising application of SAFT equations to predict the thermodynamic behavior of SCFs.
中文翻译:

兰索拉唑在超临界二氧化碳中溶解度的实验测量和热力学建模:SAFT-VR EoS 的应用
摘要 超临界流体 (SCF) 中固体溶质的溶解度测量被认为是通过 SCF 技术生产微米和纳米颗粒的主要步骤。在这项工作中,兰索拉唑 (LPZ) 在超临界二氧化碳 (SC-CO2) 中的溶解度首次分别在 12-27 MPa 和 308.2-338.2 K 的压力和温度范围内测定。在应用条件下,摩尔分数在 1.15 × 10-5 到 7.36 × 10-4 的范围内。为了关联药物溶解度,应用了六个半经验模型和两个状态方程 (EoS),即 Peng-Robinson (PR) 作为立方 EoS 和 SAFT-VR 作为非立方 EoS。结果显示,在半经验模型中,“Reddy and Garlapati”模型的准确度最高,AARDz 为 7.36%。此外,AARD 为 6 的 SAFT-VR EoS。65 证明是比 PR EOS 更好的优先级,并且与所有经验模型相比显示出最高的精度。这可能是 SAFT 方程在预测 SCF 热力学行为方面的一个有前景的应用。
更新日期:2020-03-01
中文翻译:

兰索拉唑在超临界二氧化碳中溶解度的实验测量和热力学建模:SAFT-VR EoS 的应用
摘要 超临界流体 (SCF) 中固体溶质的溶解度测量被认为是通过 SCF 技术生产微米和纳米颗粒的主要步骤。在这项工作中,兰索拉唑 (LPZ) 在超临界二氧化碳 (SC-CO2) 中的溶解度首次分别在 12-27 MPa 和 308.2-338.2 K 的压力和温度范围内测定。在应用条件下,摩尔分数在 1.15 × 10-5 到 7.36 × 10-4 的范围内。为了关联药物溶解度,应用了六个半经验模型和两个状态方程 (EoS),即 Peng-Robinson (PR) 作为立方 EoS 和 SAFT-VR 作为非立方 EoS。结果显示,在半经验模型中,“Reddy and Garlapati”模型的准确度最高,AARDz 为 7.36%。此外,AARD 为 6 的 SAFT-VR EoS。65 证明是比 PR EOS 更好的优先级,并且与所有经验模型相比显示出最高的精度。这可能是 SAFT 方程在预测 SCF 热力学行为方面的一个有前景的应用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号