当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of Zea mays coniferyl aldehyde dehydrogenase by daidzin: A potential approach for the investigation of lignocellulose recalcitrance
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.procbio.2019.11.024 Ana Paula Ferro , Rogério Flores Júnior , Aline Finger-Teixeira , Angela Valderrama Parizotto , Jennifer Munik Bevilaqua , Dyoni Matias de Oliveira , Hugo Bruno Correa Molinari , Rogério Marchiosi , Wanderley Dantas dos Santos , Flávio Augusto Vicente Seixas , Osvaldo Ferrarese-Filho
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.procbio.2019.11.024 Ana Paula Ferro , Rogério Flores Júnior , Aline Finger-Teixeira , Angela Valderrama Parizotto , Jennifer Munik Bevilaqua , Dyoni Matias de Oliveira , Hugo Bruno Correa Molinari , Rogério Marchiosi , Wanderley Dantas dos Santos , Flávio Augusto Vicente Seixas , Osvaldo Ferrarese-Filho
|
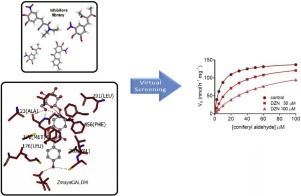
Abstract Coniferyl aldehyde dehydrogenase (CALDH) catalyzes the oxidation of coniferyl aldehyde to ferulic acid. Because ferulic acid has a relevant role in the structure and recalcitrance of the cell wall, inhibition of CALDH can reduce its levels and increase the digestibility of lignocellulosic biomass. We prospected in silico a selective inhibitor of CALDH of Zea mays. The ZmaysCALDH gene was identified by homology with the corresponding gene of Arabidopsis thaliana. The sequence was translated and analyzed, and the quaternary structure was modeled. A set of 20 putative inhibitors were screened from a virtual library and docked in the active site of ZmaysCALDH, and daidzin (DZN) was selected as an enzyme inhibitor. The stability of the ZmaysCALDH–DZN complex was evaluated by molecular dynamics simulations of the monomeric and tetrameric forms. For evaluation of kinetic analysis, ZmaysCALDH activity was determined in vitro by high-performance liquid chromatography. In comparison to the DZN-free control, the data obtained indicated constant Vmax and enhanced Km. Altogether, in silico and in vitro findings indicated that DZN inhibited ZmaysCALDH competitively. The DZN-induced inhibition of ZmaysCALDH could be a valuable and promising approach to studies on ferulic acid biosynthesis and saccharification of lignocellulosic biomass.
中文翻译:

黄豆苷对玉米松柏醛脱氢酶的抑制作用:研究木质纤维素顽固性的潜在方法
摘要 松柏醛脱氢酶 (CALDH) 催化松柏醛氧化为阿魏酸。因为阿魏酸在细胞壁的结构和顽固性中具有相关作用,抑制 CALDH 可以降低其水平并增加木质纤维素生物质的消化率。我们在计算机上预测了玉米 CALDH 的选择性抑制剂。ZmaysCALDH基因通过与拟南芥相应基因的同源性鉴定。对序列进行翻译和分析,并对四级结构进行建模。从虚拟文库中筛选出一组20种推定的抑制剂,停靠在ZmaysCALDH的活性位点,并选择黄豆苷(DZN)作为酶抑制剂。ZmaysCALDH-DZN 复合物的稳定性通过单体和四聚体形式的分子动力学模拟进行评估。为了评价动力学分析,ZmaysCALDH 活性是通过高效液相色谱法在体外测定的。与不含 DZN 的对照相比,获得的数据表明 Vmax 恒定,Km 增强。总之,计算机和体外研究结果表明,DZN 竞争性地抑制了 ZmaysCALDH。DZN 诱导的 ZmaysCALDH 抑制可能是研究阿魏酸生物合成和木质纤维素生物质糖化的一种有价值且有前景的方法。
更新日期:2020-03-01
中文翻译:

黄豆苷对玉米松柏醛脱氢酶的抑制作用:研究木质纤维素顽固性的潜在方法
摘要 松柏醛脱氢酶 (CALDH) 催化松柏醛氧化为阿魏酸。因为阿魏酸在细胞壁的结构和顽固性中具有相关作用,抑制 CALDH 可以降低其水平并增加木质纤维素生物质的消化率。我们在计算机上预测了玉米 CALDH 的选择性抑制剂。ZmaysCALDH基因通过与拟南芥相应基因的同源性鉴定。对序列进行翻译和分析,并对四级结构进行建模。从虚拟文库中筛选出一组20种推定的抑制剂,停靠在ZmaysCALDH的活性位点,并选择黄豆苷(DZN)作为酶抑制剂。ZmaysCALDH-DZN 复合物的稳定性通过单体和四聚体形式的分子动力学模拟进行评估。为了评价动力学分析,ZmaysCALDH 活性是通过高效液相色谱法在体外测定的。与不含 DZN 的对照相比,获得的数据表明 Vmax 恒定,Km 增强。总之,计算机和体外研究结果表明,DZN 竞争性地抑制了 ZmaysCALDH。DZN 诱导的 ZmaysCALDH 抑制可能是研究阿魏酸生物合成和木质纤维素生物质糖化的一种有价值且有前景的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号