当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Base-controlled product switch in the ruthenium-catalyzed protodecarbonylation of phthalimides: a mechanistic study
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019-11-21 , DOI: 10.1039/c9cy02047k Massimo Christian D'Alterio 1, 2, 3, 4, 5 , Yu-Chao Yuan 6, 7, 8, 9, 10 , Christian Bruneau 6, 7, 8, 9, 10 , Giovanni Talarico 11, 12, 13, 14 , Rafael Gramage-Doria 6, 7, 8, 9, 10 , Albert Poater 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019-11-21 , DOI: 10.1039/c9cy02047k Massimo Christian D'Alterio 1, 2, 3, 4, 5 , Yu-Chao Yuan 6, 7, 8, 9, 10 , Christian Bruneau 6, 7, 8, 9, 10 , Giovanni Talarico 11, 12, 13, 14 , Rafael Gramage-Doria 6, 7, 8, 9, 10 , Albert Poater 1, 2, 3, 4, 5
Affiliation
|
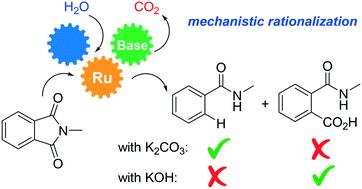
The whole reaction mechanism of the ruthenium-catalyzed protodecarbonylation of N-substituted phthalimides into secondary amides was unravelled by a combined experimental and theoretical study. The chemoselectivity of the reaction, which is catalyzed by para-cymene coordinated Ru(II) species all over the catalytic cycle, is exclusively controlled by the unique roles of the bases. Meanwhile, in the presence of K2CO3 or KOH at high temperatures, the same product (benzamide) is mainly formed, whereas at low temperatures, KOH led to an unexpected side-product (phthalamic acid) and no reactivity was observed with K2CO3. The non-covalent interactions between the potassium cations and the different carbonyl groups in the molecules are key to providing a thermodynamically favourable pathway with energetically accessible transition states. The unexpected formation of carbon dioxide (CO2) in the course of the reaction originates from the phthalimide substrate and the base K2CO3 in two different elementary steps, respectively.
中文翻译:

钌催化的邻苯二甲酰亚胺原羰基化反应中的碱控制产物转换:机理研究
结合实验和理论研究,揭示了钌催化的N-取代的邻苯二甲酰亚胺的原羰基羰基化反应生成仲酰胺的整个反应机理。在整个催化循环中,由对伞花烯配位的Ru(II)物种催化的反应的化学选择性仅受碱的独特作用控制。同时,在高温下存在K 2 CO 3或KOH时,主要形成相同的产物(苯甲酰胺),而在低温下,KOH导致意外的副产物(邻苯二甲酸),并且未观察到与K的反应性。2一氧化碳3。钾阳离子和分子中不同羰基之间的非共价相互作用是提供具有能量可及的过渡态的热力学上有利的途径的关键。反应过程中意外产生的二氧化碳(CO 2)分别来自邻苯二甲酰亚胺底物和碱K 2 CO 3的两个不同基本步骤。
更新日期:2019-11-21
中文翻译:

钌催化的邻苯二甲酰亚胺原羰基化反应中的碱控制产物转换:机理研究
结合实验和理论研究,揭示了钌催化的N-取代的邻苯二甲酰亚胺的原羰基羰基化反应生成仲酰胺的整个反应机理。在整个催化循环中,由对伞花烯配位的Ru(II)物种催化的反应的化学选择性仅受碱的独特作用控制。同时,在高温下存在K 2 CO 3或KOH时,主要形成相同的产物(苯甲酰胺),而在低温下,KOH导致意外的副产物(邻苯二甲酸),并且未观察到与K的反应性。2一氧化碳3。钾阳离子和分子中不同羰基之间的非共价相互作用是提供具有能量可及的过渡态的热力学上有利的途径的关键。反应过程中意外产生的二氧化碳(CO 2)分别来自邻苯二甲酰亚胺底物和碱K 2 CO 3的两个不同基本步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号