当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Distinct α-Synuclein:Lipid Co-Structure Complexes Affect Amyloid Nucleation through Fibril Mimetic Behavior.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.biochem.9b00925 Ersoy Cholak 1 , Saskia Bucciarelli 1 , Katrine Bugge 2 , Nicolai Tidemand Johansen 3 , Bente Vestergaard 1 , Lise Arleth 3 , Birthe B Kragelund 2 , Annette E Langkilde 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.biochem.9b00925 Ersoy Cholak 1 , Saskia Bucciarelli 1 , Katrine Bugge 2 , Nicolai Tidemand Johansen 3 , Bente Vestergaard 1 , Lise Arleth 3 , Birthe B Kragelund 2 , Annette E Langkilde 1
Affiliation
|
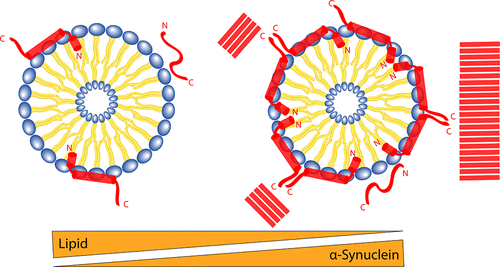
A hallmark of Parkinson's disease is the presence of Lewy bodies consisting of lipids and proteins, mainly fibrillated α-synuclein (aSN). aSN is an intrinsically disordered protein exerting its physiological role in an ensemble of states, one of which coexists in large assemblies with lipids, recently termed co-structures. Here, we decipher the kinetics of aSN:lipid co-structure formation to decode its mechanism of formation, and we show that the co-structures form with a distinct stoichiometry. Through seeded fibrillation assays, we demonstrate that aSN:lipid co-structures accelerate aSN fibril nucleation compared to lipid vesicles alone. A small-angle X-ray scattering-based model is proposed in which aSN decorates the lipid vesicle surface, yielding properties similar to those of the fibril surface, enhancing fibril nucleation. The delicate balance of aSN structural states close to and on the membrane may under given conditions, e.g., increased local concentrations, be a crucial switching factor between functional and pathological behavior.
中文翻译:

不同的α-突触核蛋白:脂质共结构复合物通过原纤维模拟行为影响淀粉样蛋白成核。
帕金森氏病的标志是路易体的存在,其由脂质和蛋白质组成,主要是原纤维化的α-突触核蛋白(aSN)。aSN是一种内在失调的蛋白质,在一组状态中发挥其生理作用,其中一种状态与脂质(大体上共存)共存,最近被称为共结构。在这里,我们破译aSN:脂质共结构形成的动力学,以解码其形成机理,并且我们表明该共结构形成具有不同的化学计量。通过播种原纤化试验,我们证明与单独的脂质囊泡相比,aSN:脂质共结构促进aSN原纤维成核。提出了一种基于小角度X射线散射的模型,其中aSN装饰脂质囊泡表面,产生与原纤维表面相似的特性,从而增强了原纤维的成核作用。
更新日期:2019-12-04
中文翻译:

不同的α-突触核蛋白:脂质共结构复合物通过原纤维模拟行为影响淀粉样蛋白成核。
帕金森氏病的标志是路易体的存在,其由脂质和蛋白质组成,主要是原纤维化的α-突触核蛋白(aSN)。aSN是一种内在失调的蛋白质,在一组状态中发挥其生理作用,其中一种状态与脂质(大体上共存)共存,最近被称为共结构。在这里,我们破译aSN:脂质共结构形成的动力学,以解码其形成机理,并且我们表明该共结构形成具有不同的化学计量。通过播种原纤化试验,我们证明与单独的脂质囊泡相比,aSN:脂质共结构促进aSN原纤维成核。提出了一种基于小角度X射线散射的模型,其中aSN装饰脂质囊泡表面,产生与原纤维表面相似的特性,从而增强了原纤维的成核作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号