当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Reactions of Cationic Metallofullerenes: An Alternative Route for Exohedral Functionalization.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-01-24 , DOI: 10.1002/chem.201904854 Yajing Hu 1 , Albert Solé-Daura 2 , Yang-Rong Yao 3 , Xuechen Liu 1 , Sijie Liu 1 , Ao Yu 1 , Ping Peng 1 , Josep M Poblet 2 , Antonio Rodríguez-Fortea 2 , Luis Echegoyen 3 , Fang-Fang Li 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-01-24 , DOI: 10.1002/chem.201904854 Yajing Hu 1 , Albert Solé-Daura 2 , Yang-Rong Yao 3 , Xuechen Liu 1 , Sijie Liu 1 , Ao Yu 1 , Ping Peng 1 , Josep M Poblet 2 , Antonio Rodríguez-Fortea 2 , Luis Echegoyen 3 , Fang-Fang Li 1
Affiliation
|
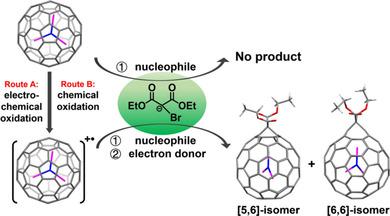
The chemistry of cationic forms of clusterfullerenes remain less explored than that of the corresponding neutral or anionic species. In the present work, M3 N@Ih -C80 (M=Sc or Lu) cations were generated by both electrochemical and chemical oxidation methods. The as-obtained cations successfully underwent the typical Bingel-Hirsch reaction that fails with neutral Sc3 N@Ih -C80 . Two isomeric Sc3 N@Ih -C80 cation derivatives, [5,6]-open and [6,6]-open adducts, were synthesized, and the former has never been prepared by means of a Bingel-Hirsch reaction with neutral clusterfullerenes. In the case of the Lu3 N@Ih -C80 cation, however, only a [6,6]-open adduct was obtained. Density functional theory (DFT) calculations indicated that the oxidized M3 N@Ih -C80 was much more reactive than the neutral compound upon addition of the diethyl bromomalonate anion. The Bingel-Hirsch reaction of M3 N@Ih -C80 cations occurred by means of an unusual outer-sphere single-electron transfer (SET) process from the diethyl bromomalonate anion to the stable intermediate [M3 N@C80 (C2 H5 COO)2 CBr]. . Remarkably, the diethyl bromomalonate anion was found to act as both a nucleophile and an electron donor.
中文翻译:

阳离子金属富勒烯的化学反应:Exohedral官能化的替代途径。
与相应的中性或阴离子物质相比,团簇富勒烯的阳离子形式的化学研究较少。在目前的工作中,M3 N @ Ih -C80(M = Sc或Lu)阳离子是通过电化学和化学氧化方法生成的。所获得的阳离子成功进行了典型的Bingel-Hirsch反应,该反应因中性Sc3 N @ Ih -C80而失败。合成了两个异构的Sc3 N @ Ih -C80阳离子衍生物,[5,6]-开放和[6,6]-开放的加合物,而前者从未通过与中性簇富勒烯的Bingel-Hirsch反应制备。但是,在Lu3 N @ Ih -C80阳离子的情况下,仅获得[6,6]-开放的加合物。密度泛函理论(DFT)计算表明,添加溴代丙二酸二乙酯阴离子后,氧化的M3 N @ Ih -C80的反应性比中性化合物高得多。M3 N @ Ih -C80阳离子的Bingel-Hirsch反应是通过不寻常的外球单电子转移(SET)过程从溴代丙二酸二乙酯阴离子转移到稳定的中间体[M3 N @ C80(C2 H5 COO)2 CBr]。。值得注意的是,发现溴代丙二酸二乙酯阴离子既可以充当亲核试剂,也可以充当电子给体。
更新日期:2020-01-24
中文翻译:

阳离子金属富勒烯的化学反应:Exohedral官能化的替代途径。
与相应的中性或阴离子物质相比,团簇富勒烯的阳离子形式的化学研究较少。在目前的工作中,M3 N @ Ih -C80(M = Sc或Lu)阳离子是通过电化学和化学氧化方法生成的。所获得的阳离子成功进行了典型的Bingel-Hirsch反应,该反应因中性Sc3 N @ Ih -C80而失败。合成了两个异构的Sc3 N @ Ih -C80阳离子衍生物,[5,6]-开放和[6,6]-开放的加合物,而前者从未通过与中性簇富勒烯的Bingel-Hirsch反应制备。但是,在Lu3 N @ Ih -C80阳离子的情况下,仅获得[6,6]-开放的加合物。密度泛函理论(DFT)计算表明,添加溴代丙二酸二乙酯阴离子后,氧化的M3 N @ Ih -C80的反应性比中性化合物高得多。M3 N @ Ih -C80阳离子的Bingel-Hirsch反应是通过不寻常的外球单电子转移(SET)过程从溴代丙二酸二乙酯阴离子转移到稳定的中间体[M3 N @ C80(C2 H5 COO)2 CBr]。。值得注意的是,发现溴代丙二酸二乙酯阴离子既可以充当亲核试剂,也可以充当电子给体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号