当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Positively Charged Residues in the Head Domain of P2X4 Receptors Assist the Binding of ATP.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.jcim.9b00856 Vanesa Racigh 1, 2 , Agustín Ormazábal 1, 2 , Juliana Palma 1, 2 , Gustavo Pierdominici-Sottile 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.jcim.9b00856 Vanesa Racigh 1, 2 , Agustín Ormazábal 1, 2 , Juliana Palma 1, 2 , Gustavo Pierdominici-Sottile 1, 2
Affiliation
|
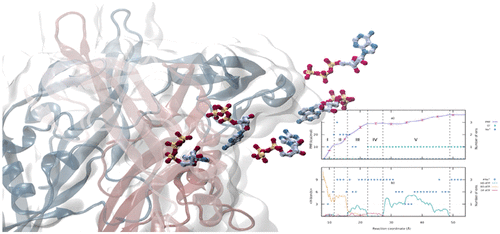
P2X receptors are a family of trimeric cationic channels located in the membrane of mammalian cells. They open in response to the binding of ATP. The differences between the closed and open structures have been described in detail for some members of the family. However, the order in which the conformational changes take place as ATP enters the binding cleft, and the residues involved in the intermediate stages, are still unknown. Here, we present the results of umbrella sampling simulations aimed to elucidate the sequence of conformational changes that occur during the reversible binding of ATP to the P2X4 receptor. The simulations also provided information about the interactions that develop in the course of the process. In particular, they revealed the existence of a metastable state which assists the binding. This state is stabilized by positively charged residues located in the head domain of the receptor. Based on these findings, we propose a novel mechanism for the capture of ATP by P2X4 receptors.
中文翻译:

P2X4受体的头部域中带正电荷的残基有助于ATP的结合。
P2X受体是位于哺乳动物细胞膜上的三聚阳离子通道家族。它们打开以响应ATP的结合。对于家庭中的某些成员,已经详细描述了封闭结构和开放结构之间的差异。但是,随着ATP进入结合裂隙,构象变化的顺序以及中间阶段涉及的残基仍然未知。在这里,我们介绍了伞状采样模拟的结果,旨在阐明在ATP与P2X4受体可逆结合过程中发生的构象变化序列。模拟还提供了有关在过程中发展的交互作用的信息。特别地,他们揭示了辅助结合的亚稳状态的存在。这种状态通过位于受体头部结构域中的带正电荷的残基得以稳定。基于这些发现,我们提出了一种通过P2X4受体捕获ATP的新机制。
更新日期:2020-01-14
中文翻译:

P2X4受体的头部域中带正电荷的残基有助于ATP的结合。
P2X受体是位于哺乳动物细胞膜上的三聚阳离子通道家族。它们打开以响应ATP的结合。对于家庭中的某些成员,已经详细描述了封闭结构和开放结构之间的差异。但是,随着ATP进入结合裂隙,构象变化的顺序以及中间阶段涉及的残基仍然未知。在这里,我们介绍了伞状采样模拟的结果,旨在阐明在ATP与P2X4受体可逆结合过程中发生的构象变化序列。模拟还提供了有关在过程中发展的交互作用的信息。特别地,他们揭示了辅助结合的亚稳状态的存在。这种状态通过位于受体头部结构域中的带正电荷的残基得以稳定。基于这些发现,我们提出了一种通过P2X4受体捕获ATP的新机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号