当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Specific Conjugation of the Indolinobenzodiazepine DGN549 to Antibodies Affords Antibody-Drug Conjugates with an Improved Therapeutic Index as Compared with Lysine Conjugation.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2019-12-10 , DOI: 10.1021/acs.bioconjchem.9b00777 Chen Bai 1 , Emily E Reid 1 , Alan Wilhelm 1 , Manami Shizuka 1 , Erin K Maloney 1 , Rassol Laleau 1 , Lauren Harvey 1 , Katie E Archer 1 , Dilrukshi Vitharana 1 , Sharlene Adams 1 , Yelena Kovtun 1 , Michael L Miller 1 , Ravi Chari 1 , Thomas A Keating 1 , Nicholas C Yoder 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2019-12-10 , DOI: 10.1021/acs.bioconjchem.9b00777 Chen Bai 1 , Emily E Reid 1 , Alan Wilhelm 1 , Manami Shizuka 1 , Erin K Maloney 1 , Rassol Laleau 1 , Lauren Harvey 1 , Katie E Archer 1 , Dilrukshi Vitharana 1 , Sharlene Adams 1 , Yelena Kovtun 1 , Michael L Miller 1 , Ravi Chari 1 , Thomas A Keating 1 , Nicholas C Yoder 1
Affiliation
|
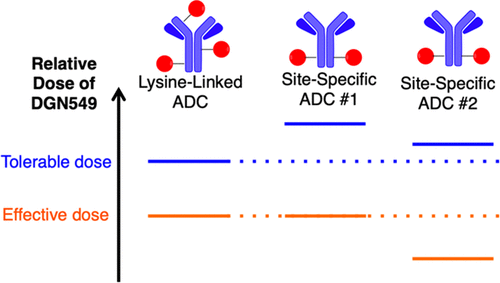
Antibody-drug conjugates have elicited great interest recently as targeted chemotherapies for cancer. Recent preclinical and clinical data have continued to raise questions about optimizing the design of these complex therapeutics. Biochemical methods for site-specific antibody conjugation have been a design feature of recent clinical ADCs, and preclinical reports suggest that site-specifically conjugated ADCs generically offer improved therapeutic indices (i.e., the fold difference between efficacious and maximum tolerated doses). Here we present the results of a systematic preclinical comparison of ADCs embodying the DNA-alkylating linker-payload DGN549 generated with both heterogeneous lysine-directed and site-specific cysteine-directed conjugation chemistries. Importantly, the catabolites generated by each ADC are the same regardless of the conjugation format. In two different model systems evaluated, the site-specific ADC showed a therapeutic index benefit. However, the therapeutic index benefit is different in each case: both show evidence of improved tolerability, though with different magnitudes, and in one case significant efficacy improvement is also observed. These results support our contention that conjugation chemistry of ADCs is best evaluated in the context of a particular antibody, target, and linker-payload, and ideally across multiple disease models.
中文翻译:

吲哚苯并二氮卓类DGN549与抗体Affords抗体-药物缀合物的位点特异性缀合物与赖氨酸缀合物相比具有更高的治疗指数。
抗体-药物缀合物作为癌症的靶向化学疗法最近引起了极大的兴趣。最近的临床前和临床数据继续引起关于优化这些复杂疗法的设计的问题。用于位点特异性抗体缀合的生化方法已成为最近临床ADC的设计特征,并且临床前报告表明,位点特异性缀合的ADC通常可提供改善的治疗指数(即有效剂量与最大耐受剂量之间的倍数差异)。在这里,我们介绍了ADC的系统化的临床前比较结果,这些ADC体现了用异源赖氨酸定向和位点特异性半胱氨酸定向偶联化学生成的DNA烷基化接头有效负载DGN549。重要的,不管共轭形式如何,每个ADC产生的分解代谢物都是相同的。在评估的两个不同模型系统中,位点特异性ADC显示出治疗指标的优势。但是,每种情况下的治疗指数收益均不同:尽管大小不同,但两者均显示出改善的耐受性的证据,并且在一种情况下,还观察到了显着的疗效改善。这些结果支持了我们的观点,即在特定抗体,靶标和接头有效负载的情况下,最好在多种疾病模型中评估ADC的共轭化学。尽管具有不同的幅度,但在一种情况下,也观察到明显的功效改善。这些结果支持了我们的观点,即在特定抗体,靶标和接头有效负载的情况下,最好在多种疾病模型中评估ADC的共轭化学。尽管具有不同的幅度,但在一种情况下,也观察到了显着的功效改善。这些结果支持了我们的观点,即在特定抗体,靶标和接头有效负载的情况下,最好在多种疾病模型中评估ADC的共轭化学。
更新日期:2019-12-11
中文翻译:

吲哚苯并二氮卓类DGN549与抗体Affords抗体-药物缀合物的位点特异性缀合物与赖氨酸缀合物相比具有更高的治疗指数。
抗体-药物缀合物作为癌症的靶向化学疗法最近引起了极大的兴趣。最近的临床前和临床数据继续引起关于优化这些复杂疗法的设计的问题。用于位点特异性抗体缀合的生化方法已成为最近临床ADC的设计特征,并且临床前报告表明,位点特异性缀合的ADC通常可提供改善的治疗指数(即有效剂量与最大耐受剂量之间的倍数差异)。在这里,我们介绍了ADC的系统化的临床前比较结果,这些ADC体现了用异源赖氨酸定向和位点特异性半胱氨酸定向偶联化学生成的DNA烷基化接头有效负载DGN549。重要的,不管共轭形式如何,每个ADC产生的分解代谢物都是相同的。在评估的两个不同模型系统中,位点特异性ADC显示出治疗指标的优势。但是,每种情况下的治疗指数收益均不同:尽管大小不同,但两者均显示出改善的耐受性的证据,并且在一种情况下,还观察到了显着的疗效改善。这些结果支持了我们的观点,即在特定抗体,靶标和接头有效负载的情况下,最好在多种疾病模型中评估ADC的共轭化学。尽管具有不同的幅度,但在一种情况下,也观察到明显的功效改善。这些结果支持了我们的观点,即在特定抗体,靶标和接头有效负载的情况下,最好在多种疾病模型中评估ADC的共轭化学。尽管具有不同的幅度,但在一种情况下,也观察到了显着的功效改善。这些结果支持了我们的观点,即在特定抗体,靶标和接头有效负载的情况下,最好在多种疾病模型中评估ADC的共轭化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号