当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein knots provide mechano-resilience to an AAA+ protease-mediated proteolysis with profound ATP energy expenses.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-11-20 , DOI: 10.1016/j.bbapap.2019.140330 Manoj Kumar Sriramoju,Yen Chen,Shang-Te Danny Hsu
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-11-20 , DOI: 10.1016/j.bbapap.2019.140330 Manoj Kumar Sriramoju,Yen Chen,Shang-Te Danny Hsu
|
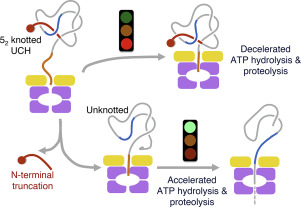
Knotted proteins are some of the most fascinating examples of how linear polypeptide chains can achieve intricate topological arrangements efficiently and spontaneously. The entanglements of polypeptide chains could potentially enhance their folding stabilities. We recently reported the unprecedented mechanostability of the Gordian (52) knotted family of human ubiquitin C-terminal hydrolases (UCHs) in the context of withstanding the mechanical unfolding of the bacterial AAA+ proteasome, ClpXP; a green fluorescence protein (GFP) was fused to the N-terminus of various UCHs as a reporter of the unfolding and degradation of these topologically knotted substrates, but it also limited the ability to examine the effect of untying the knotted topology via N-terminal truncation. In this study, we directly monitored the ClpXP-mediated degradation of UCH variants by electrophoresis and quantitative imaging analyses. We demonstrated that untying of the 52 knot in UCHL1 via N-terminal truncation (UCHL1Δ11) significantly reduces its mechanostability. We further quantified the ATP expenditures of degrading different UCH variants by ClpXP. The unknotted UCHL1Δ11 underwent accelerated ClpXP-dependent proteolysis, with a 30-fold reduction in ATP consumption compared to the knotted wild type. Unlike all other known ClpXP substrates, UCHL5, which is the most resilient substrate known to date, significantly slowed down the ATP turnover rate by ClpXP. Furthermore, UCHL5 required 1000-fold more ATP to be fully degraded by ClpXP compared to GFP. Our results underscored how the complex, knotted folding topology in UCHs may interfere with the mechano-unfolding processes of the AAA+ unfoldase, ClpX.
中文翻译:

蛋白质结可为AAA +蛋白酶介导的蛋白水解提供机械弹性,同时消耗大量的ATP能量。
打结的蛋白质是线性多肽链如何有效并自发地实现复杂拓扑结构的一些最引人入胜的例子。多肽链的缠结可能会增强其折叠稳定性。我们最近报道了在抵抗细菌AAA +蛋白酶体ClpXP的机械展开的背景下,人遍在蛋白C末端水解酶(UCH)的高迪安(52)打结家族史无前例的可机械性。将绿色荧光蛋白(GFP)融合到各种UCH的N端,作为这些拓扑结底物展开和降解的报告基因,但它也限制了通过N端检查解结拓扑的效果的能力。截断。在这项研究中,我们通过电泳和定量成像分析直接监测了ClpXP介导的UCH变体的降解。我们证明,通过N端截短(UCHL1Δ11)可以使UCHL1中的52个结解开,从而显着降低其机械稳定性。我们进一步量化了ClpXP降解不同UCH变体的ATP支出。未打结的UCHL1Δ11经历了加速的ClpXP依赖性蛋白水解,与打结的野生型相比,ATP消耗降低了30倍。与所有其他已知的ClpXP底物不同,UCHL5是迄今为止已知的最具弹性的底物,它大大降低了ClpXP的ATP周转率。此外,与GFP相比,UCHL5需要ClpXP完全降解的ATP多出1000倍。我们的结果强调了复杂性,
更新日期:2019-11-20
中文翻译:

蛋白质结可为AAA +蛋白酶介导的蛋白水解提供机械弹性,同时消耗大量的ATP能量。
打结的蛋白质是线性多肽链如何有效并自发地实现复杂拓扑结构的一些最引人入胜的例子。多肽链的缠结可能会增强其折叠稳定性。我们最近报道了在抵抗细菌AAA +蛋白酶体ClpXP的机械展开的背景下,人遍在蛋白C末端水解酶(UCH)的高迪安(52)打结家族史无前例的可机械性。将绿色荧光蛋白(GFP)融合到各种UCH的N端,作为这些拓扑结底物展开和降解的报告基因,但它也限制了通过N端检查解结拓扑的效果的能力。截断。在这项研究中,我们通过电泳和定量成像分析直接监测了ClpXP介导的UCH变体的降解。我们证明,通过N端截短(UCHL1Δ11)可以使UCHL1中的52个结解开,从而显着降低其机械稳定性。我们进一步量化了ClpXP降解不同UCH变体的ATP支出。未打结的UCHL1Δ11经历了加速的ClpXP依赖性蛋白水解,与打结的野生型相比,ATP消耗降低了30倍。与所有其他已知的ClpXP底物不同,UCHL5是迄今为止已知的最具弹性的底物,它大大降低了ClpXP的ATP周转率。此外,与GFP相比,UCHL5需要ClpXP完全降解的ATP多出1000倍。我们的结果强调了复杂性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号