当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multikilogram Synthesis of a Potent Dual Bcl-2/Bcl-xL Antagonist. 2. Manufacture of the 1,3-Diamine Moiety and Improvement of the Final Coupling Reaction
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-11-19 , DOI: 10.1021/acs.oprd.9b00367 Christophe Hardouin 1 , Sandrine Baillard 1 , François Barière 1 , Anthony Craquelin 1 , Mathieu Grandjean 1 , Solenn Janvier 1 , Stéphane Le Roux 1 , Christine Penloup 1 , Olivier Russo 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-11-19 , DOI: 10.1021/acs.oprd.9b00367 Christophe Hardouin 1 , Sandrine Baillard 1 , François Barière 1 , Anthony Craquelin 1 , Mathieu Grandjean 1 , Solenn Janvier 1 , Stéphane Le Roux 1 , Christine Penloup 1 , Olivier Russo 1
Affiliation
|
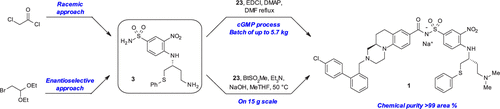
This paper describes the synthesis of kilogram quantities of the sulfonamide moiety 3 involved in a coupling reaction with acid moiety 2 to provide batches of drug candidate 1 for preclinical studies and first-in-human clinical trials. A first approach relying on a chiral separation furnished the desired enantiomer of 1,3-diamine 20, precursor of sulfonamide 3. An enantiomeric synthesis of 20 using the Ellman’s chiral auxiliary coupled with an aza-Reformatsky reaction to control the stereochemistry is also discussed. Coupling conditions of the final step involving EDCI to provide 1 under a cGMP process are detailed. An alternative approach using N-(1-methanesulfonyl)benzotriazole is also presented.
中文翻译:

强大的双重Bcl-2 / Bcl-x L拮抗剂的多千克合成。2. 1,3-二胺部分的制造和最终偶联反应的改进
本文描述了与酸部分2偶联反应中涉及的千克量磺酰胺部分3的合成,以提供批次的候选药物1用于临床前研究和首次人类临床试验。依靠手性分离的第一种方法提供了所需的1,3-二胺20(磺酰胺3的前体)对映体。还讨论了使用Ellman手性助剂与aza-Reformatsky反应耦合以控制立体化学的对映体合成20。详细介绍了EDCI在cGMP流程中提供1的最终步骤的耦合条件。另一种使用方法还提出了N-(1-甲磺酰基)苯并三唑。
更新日期:2019-11-19
中文翻译:

强大的双重Bcl-2 / Bcl-x L拮抗剂的多千克合成。2. 1,3-二胺部分的制造和最终偶联反应的改进
本文描述了与酸部分2偶联反应中涉及的千克量磺酰胺部分3的合成,以提供批次的候选药物1用于临床前研究和首次人类临床试验。依靠手性分离的第一种方法提供了所需的1,3-二胺20(磺酰胺3的前体)对映体。还讨论了使用Ellman手性助剂与aza-Reformatsky反应耦合以控制立体化学的对映体合成20。详细介绍了EDCI在cGMP流程中提供1的最终步骤的耦合条件。另一种使用方法还提出了N-(1-甲磺酰基)苯并三唑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号