当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theory Demonstrated a “Coupled” Mechanism for O2 Activation and Substrate Hydroxylation by Binuclear Copper Monooxygenases
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-11-20 , DOI: 10.1021/jacs.9b09172 Peng Wu 1, 2 , Fangfang Fan 3 , Jinshuai Song 4 , Wei Peng 3 , Jia Liu 3 , Chunsen Li 1, 5 , Zexing Cao 3 , Binju Wang 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-11-20 , DOI: 10.1021/jacs.9b09172 Peng Wu 1, 2 , Fangfang Fan 3 , Jinshuai Song 4 , Wei Peng 3 , Jia Liu 3 , Chunsen Li 1, 5 , Zexing Cao 3 , Binju Wang 3
Affiliation
|
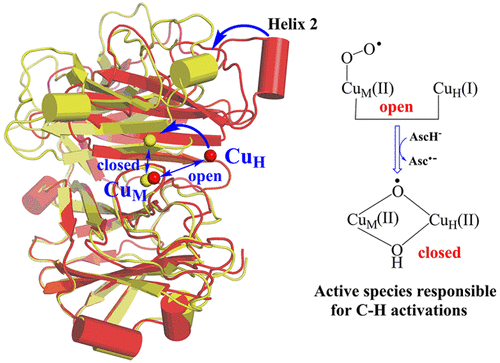
Multiscale simulations have been performed to address the longstanding issue of the "dioxygen activation" by the binucle-ar copper monooxygenases (PHM and DβM), which were traditionally classified as the "noncoupled" binuclear copper enzymes. Our QM/MM calculations rule out that CuM(II)-O2• is an active species for H-abstraction from the substrate. By contrast, CuM(II)-O2• would abstract an H atom from the co-substrate ascorbate to form a CuM(II)-OOH intermediate in PHM and DβM. Consistent with the recently reported structural features of DβM, the umbrella sampling shows that the "open" conformation of CuM(II)-OOH intermediate could readily transform into the "closed" conformation in PHM, in which we located a mixed-valent μ-hydroperoxodicopper(I,II) intermediate, (μ-OOH)Cu(I)Cu(II). The subsequent O-O cleavage and OH moiety migration to CuH generate an unexpected species of (μ-O)(μ-OH)Cu(II)Cu(II), which is revealed to be the reactive intermediate responsible for substrate hydroxylation. We also demonstrate that the flexible Met ligand is favorable for O-O cleavage reaction, while the replacement of Met with the strongly bound His ligand would inhibit the O-O cleavage reactivity. As such, the study not only demonstrates a "coupled" mechanism for O2 activation by binuclear cop-per monooxygenases, but also deciphers the full catalytic cycle of PHM and DβM in accord with the available experimental data. These findings of O2 activation and substrate hydroxylation by binuclear copper monooxygenases could expand our understandings on the reactivities of the synthetic mono-copper complexes.
中文翻译:

理论证明了双核铜单加氧酶对 O2 活化和底物羟基化的“耦合”机制
已经进行了多尺度模拟来解决双核-ar 铜单加氧酶(PHM 和 DβM)的“双氧活化”的长期问题,传统上将其归类为“非耦合”双核铜酶。我们的 QM/MM 计算排除了 CuM(II)-O2• 是从底物吸氢的活性物质。相比之下,CuM(II)-O2• 会从共底物抗坏血酸盐中提取一个 H 原子,在 PHM 和 DβM 中形成 CuM(II)-OOH 中间体。与最近报道的 DβM 结构特征一致,伞形采样表明 CuM(II)-OOH 中间体的“开放”构象可以很容易地转化为 PHM 中的“闭合”构象,我们在其中找到了一个混合价的 μ-氢过氧化二铜(I,II)中间体,(μ-OOH)Cu(I)Cu(II)。随后的 OO 裂解和 OH 部分迁移到 CuH 产生了一种意想不到的 (μ-O)(μ-OH)Cu(II)Cu(II),它被揭示是负责底物羟基化的反应性中间体。我们还证明灵活的 Met 配体有利于 OO 裂解反应,而用强结合的 His 配体替换 Met 会抑制 OO 裂解反应。因此,该研究不仅证明了双核铜单加氧酶激活 O2 的“耦合”机制,而且还根据可用的实验数据破译了 PHM 和 DβM 的完整催化循环。双核铜单加氧酶对 O2 活化和底物羟基化的这些发现可以扩展我们对合成单铜配合物的反应性的理解。
更新日期:2019-11-20
中文翻译:

理论证明了双核铜单加氧酶对 O2 活化和底物羟基化的“耦合”机制
已经进行了多尺度模拟来解决双核-ar 铜单加氧酶(PHM 和 DβM)的“双氧活化”的长期问题,传统上将其归类为“非耦合”双核铜酶。我们的 QM/MM 计算排除了 CuM(II)-O2• 是从底物吸氢的活性物质。相比之下,CuM(II)-O2• 会从共底物抗坏血酸盐中提取一个 H 原子,在 PHM 和 DβM 中形成 CuM(II)-OOH 中间体。与最近报道的 DβM 结构特征一致,伞形采样表明 CuM(II)-OOH 中间体的“开放”构象可以很容易地转化为 PHM 中的“闭合”构象,我们在其中找到了一个混合价的 μ-氢过氧化二铜(I,II)中间体,(μ-OOH)Cu(I)Cu(II)。随后的 OO 裂解和 OH 部分迁移到 CuH 产生了一种意想不到的 (μ-O)(μ-OH)Cu(II)Cu(II),它被揭示是负责底物羟基化的反应性中间体。我们还证明灵活的 Met 配体有利于 OO 裂解反应,而用强结合的 His 配体替换 Met 会抑制 OO 裂解反应。因此,该研究不仅证明了双核铜单加氧酶激活 O2 的“耦合”机制,而且还根据可用的实验数据破译了 PHM 和 DβM 的完整催化循环。双核铜单加氧酶对 O2 活化和底物羟基化的这些发现可以扩展我们对合成单铜配合物的反应性的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号