当前位置:
X-MOL 学术
›
J. Mol. Recognit.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PDZ/PDZ interaction between PSD-95 and nNOS neuronal proteins: A thermodynamic analysis of the PSD95-PDZ2/nNOS-PDZ interaction.
Journal of Molecular Recognition ( IF 2.3 ) Pub Date : 2019-11-19 , DOI: 10.1002/jmr.2826 Javier Murciano-Calles 1 , Andrea Coello 1 , Ana Cámara-Artigas 2 , Jose C Martinez 1
Journal of Molecular Recognition ( IF 2.3 ) Pub Date : 2019-11-19 , DOI: 10.1002/jmr.2826 Javier Murciano-Calles 1 , Andrea Coello 1 , Ana Cámara-Artigas 2 , Jose C Martinez 1
Affiliation
|
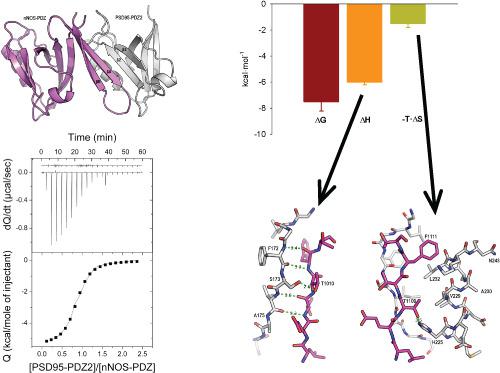
N-Methyl-D-aspartate (NMDA) receptors are key components in synaptic communication and are highly relevant in central nervous disorders, where they trigger excessive calcium entry into the neuronal cells causing harmful overproduction of nitric oxide by the neuronal nitric oxide synthase (nNOS) protein. Remarkably, NMDA receptor activation is aided by a second protein, postsynaptic density of 95 kDa (PSD95), forming the ternary protein complex NMDA/PSD95/nNOS. To minimize the potential side effects derived from blocking this ternary complex or either of its protein components, a promising approach points to the disruption of the PSD-95/nNOS interaction which is mediated by a PDZ/PDZ domain complex. Since the rational development of molecules targeting such protein-protein interaction relies on energetic and structural information herein, we include a thermodynamic and structural analysis of the PSD95-PDZ2/nNOS-PDZ. Two energetically relevant events are structurally linked to a "two-faced" or two areas of recognition between both domains. First, the assembly of a four-stranded antiparallel β-sheet between the β hairpins of nNOS and of PSD95-PDZ2, mainly enthalpic in nature, contributes 80% to the affinity. Second, binding is entropically reinforced by the hydrophobic interaction between side chains of the same nNOS β-hairpin with the side chains of α2-helix at the binding site of PSD95-PDZ2, contributing the remaining 20% of the total affinity. These results suggest strategies for the future rational design of molecules able to disrupt this complex and constitute the first exhaustive thermodynamic analysis of a PDZ/PDZ interaction.
中文翻译:

PSD-95 和 nNOS 神经元蛋白之间的 PDZ/PDZ 相互作用:PSD95-PDZ2/nNOS-PDZ 相互作用的热力学分析。
N-甲基-D-天冬氨酸 (NMDA) 受体是突触通讯的关键组成部分,与中枢神经疾病高度相关,它们触发过多的钙进入神经元细胞,导致神经元一氧化氮合酶 (nNOS) 产生有害的一氧化氮过量) 蛋白质。值得注意的是,NMDA 受体的激活得到了第二种蛋白质、95 kDa 的突触后密度 (PSD95) 的帮助,形成了三元蛋白质复合物 NMDA/PSD95/nNOS。为了最大限度地减少因阻断这种三元复合物或其任何一种蛋白质成分而产生的潜在副作用,一种有前景的方法指向了由 PDZ/PDZ 域复合物介导的 PSD-95/nNOS 相互作用的破坏。由于靶向这种蛋白质-蛋白质相互作用的分子的合理发展依赖于本文的能量和结构信息,我们包括 PSD95-PDZ2/nNOS-PDZ 的热力学和结构分析。两个能量相关的事件在结构上与两个领域之间的“双面”或两个识别领域相关联。首先,nNOS 和 PSD95-PDZ2 的 β 发夹之间的四链反平行 β 折叠的组装,主要是自然界中的焓,对亲和力贡献了 80%。其次,通过相同 nNOS β-发夹的侧链与 PSD95-PDZ2 结合位点处的 α2-螺旋侧链之间的疏水相互作用,结合熵增强,贡献总亲和力的剩余 20%。这些结果提出了未来合理设计分子的策略,能够破坏这种复合物并构成对 PDZ/PDZ 相互作用的首次详尽的热力学分析。
更新日期:2020-03-03
中文翻译:

PSD-95 和 nNOS 神经元蛋白之间的 PDZ/PDZ 相互作用:PSD95-PDZ2/nNOS-PDZ 相互作用的热力学分析。
N-甲基-D-天冬氨酸 (NMDA) 受体是突触通讯的关键组成部分,与中枢神经疾病高度相关,它们触发过多的钙进入神经元细胞,导致神经元一氧化氮合酶 (nNOS) 产生有害的一氧化氮过量) 蛋白质。值得注意的是,NMDA 受体的激活得到了第二种蛋白质、95 kDa 的突触后密度 (PSD95) 的帮助,形成了三元蛋白质复合物 NMDA/PSD95/nNOS。为了最大限度地减少因阻断这种三元复合物或其任何一种蛋白质成分而产生的潜在副作用,一种有前景的方法指向了由 PDZ/PDZ 域复合物介导的 PSD-95/nNOS 相互作用的破坏。由于靶向这种蛋白质-蛋白质相互作用的分子的合理发展依赖于本文的能量和结构信息,我们包括 PSD95-PDZ2/nNOS-PDZ 的热力学和结构分析。两个能量相关的事件在结构上与两个领域之间的“双面”或两个识别领域相关联。首先,nNOS 和 PSD95-PDZ2 的 β 发夹之间的四链反平行 β 折叠的组装,主要是自然界中的焓,对亲和力贡献了 80%。其次,通过相同 nNOS β-发夹的侧链与 PSD95-PDZ2 结合位点处的 α2-螺旋侧链之间的疏水相互作用,结合熵增强,贡献总亲和力的剩余 20%。这些结果提出了未来合理设计分子的策略,能够破坏这种复合物并构成对 PDZ/PDZ 相互作用的首次详尽的热力学分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号