当前位置:
X-MOL 学术
›
Cryst. Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Effect of Boron Forms on the Crystallization Process of Lithium Carbonate
Crystal Research and Technology ( IF 1.5 ) Pub Date : 2019-11-19 , DOI: 10.1002/crat.201900169 Pengcheng Lu 1 , Xingfu Song 1 , Hang Chen 1 , Yuzhu Sun 1 , Jianguo Yu 1
Crystal Research and Technology ( IF 1.5 ) Pub Date : 2019-11-19 , DOI: 10.1002/crat.201900169 Pengcheng Lu 1 , Xingfu Song 1 , Hang Chen 1 , Yuzhu Sun 1 , Jianguo Yu 1
Affiliation
|
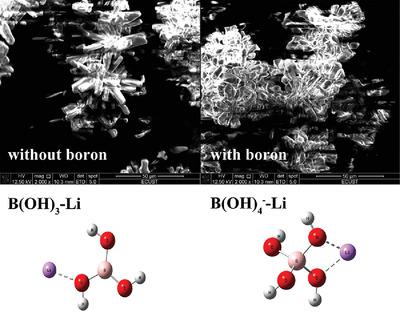
The present study investigates the effect of low concentrations of boron on the crystallization of lithium carbonate (Li2CO3). Experimental results indicate that the boron in brine mainly exists in the forms of boric acid (B(OH)3) and borate anion (B(OH)4−). Temperature and pH are critical factors to adjust the ratio of B(OH)3 and B(OH)4−. Meanwhile, the density functional theory calculation results suggest that the interaction of B(OH)4− with Li+ is stronger than that of B(OH)3 with Li+. Based on this result, the effect of boron on the crystallization of Li2CO3 is investigated and discussed at different added forms of boron, added amount of sodium hydroxide, temperatures, and concentrations of boron. B(OH)4− is proved to be the form of boron that has significant influence. Temperature and amount of sodium hydroxide have significant influence on the balance of B(OH)3 and B(OH)4−. The boron content of Li2CO3 significantly decreases as the temperature increases, and that at 80 °C is 133 ppm while that at 25 °C is 397 ppm. And that increases as nNaOH/nB increases. Finally, effect of addition of polyhydric alcohols on reducing boron entrainment is investigated.
中文翻译:

硼形态对碳酸锂结晶过程的影响
本研究研究了低浓度硼对碳酸锂(Li 2 CO 3)结晶的影响。实验结果表明,在盐水中的硼主要存在于硼酸(B(OH)的形式3)和硼酸盐阴离子(B(OH)4 - )。温度和pH是调整B(OH)的比率的关键因素3和B(OH)4 - 。同时,密度泛函理论计算结果表明,B的相互作用(OH)4 -与Li +大于B的更强(OH)3与Li +。基于该结果,研究和讨论了硼在Li的不同添加形式,氢氧化钠的添加量,温度和硼浓度下对Li 2 CO 3结晶的影响。B(OH)4 -被证明是具有显著影响硼的形式存在。温度和氢氧化钠的量对B(OH)的平衡显著影响3和B(OH)4 - 。Li 2 CO 3的硼含量随着温度的升高而显着降低,在80°C时为133 ppm,而在25°C时为397 ppm。随着n NaOH / n B的增加增加。最后,研究了添加多元醇对减少硼夹带的影响。
更新日期:2020-01-07
中文翻译:

硼形态对碳酸锂结晶过程的影响
本研究研究了低浓度硼对碳酸锂(Li 2 CO 3)结晶的影响。实验结果表明,在盐水中的硼主要存在于硼酸(B(OH)的形式3)和硼酸盐阴离子(B(OH)4 - )。温度和pH是调整B(OH)的比率的关键因素3和B(OH)4 - 。同时,密度泛函理论计算结果表明,B的相互作用(OH)4 -与Li +大于B的更强(OH)3与Li +。基于该结果,研究和讨论了硼在Li的不同添加形式,氢氧化钠的添加量,温度和硼浓度下对Li 2 CO 3结晶的影响。B(OH)4 -被证明是具有显著影响硼的形式存在。温度和氢氧化钠的量对B(OH)的平衡显著影响3和B(OH)4 - 。Li 2 CO 3的硼含量随着温度的升高而显着降低,在80°C时为133 ppm,而在25°C时为397 ppm。随着n NaOH / n B的增加增加。最后,研究了添加多元醇对减少硼夹带的影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号