当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring effects of the trifluoromethyl substituent on the chemoselectivity and regioselectivity of [3+2] cycloadditions of thiocarbonyl S‐methanides with α, β‐unsaturated ketones
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-11-19 , DOI: 10.1002/jccs.201900360 Seyed J. Hosseini 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-11-19 , DOI: 10.1002/jccs.201900360 Seyed J. Hosseini 1
Affiliation
|
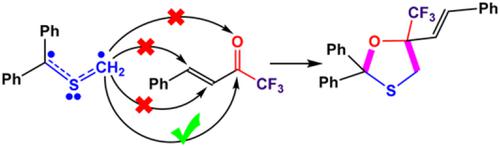
A [3+2] cycloaddition (32CA) reaction between a thiocarbonyl ylide (TCY 2) and an electron‐deficient enone (TFB 3) in tetrahydrofuran (THF) was studied in the light of molecular electron density theory at the DFT‐B3LYP/6‐31G(d) computational level to probe energetics and selectivities. The reaction was investigated in four competitive reaction paths associated with the CC and CO chemoselectivities in TFB 3. An analysis of the density functional theory‐based reactivity indices shows that TCY 2 is a strong nucleophile, and TFB 3 is also a strong electrophile. Although both C4─C5 and C6─O7 double bonds of TFB 3 can potentially be involved in 32CA reaction toward TCY 2, computed relative Gibbs free energies obviously demonstrate that C6─O7 involvement in a quite regioselective manner is entirely preferred over the C4─C5 one in an excellent agreement with the chemoselectivity and regioselectivity observed experimentally. Interestingly, such a chemoselectivity could not be rationalized through assessment of the electrophilic Parr functions calculated at the C4, C5, C6, and O7 centers of TFB 3. The global electron density transfer value, 0.31 e, calculated at the most energetically preferred transition state structure TS 1 involved within the C6─O7 chemoselective reaction channel demonstrates that this pseudodiradical type (pdr‐type) 32CA reaction has a notable polar character.
中文翻译:

探索三氟甲基取代基对硫代羰基S-甲烷与[α,β-不饱和酮] [3 + 2]环加成反应的化学选择性和区域选择性的影响
根据分子电子密度理论在DFT-B3LYP /上研究了硫代羰基内酯(TCY 2)与缺电子的烯酮(TFB 3)在四氢呋喃(THF)之间的[3 + 2]环加成反应(32CA)。6‐31G(d)的计算级别,以探讨能量和选择性。在与TFB 3中的CC和CO化学选择性相关的四个竞争性反应路径中研究了该反应。对基于密度泛函理论的反应性指数进行的分析显示,TCY 2是强亲核试剂,而TFB 3也是强亲电试剂。尽管TFB 3的C4-C5和C6-O7双键都可能参与32CA对TCY 2的反应,计算出的相对吉布斯自由能显然证明,以完全区域选择性的方式参与C6-O7完全优于C4-C5区域,这与实验观察到的化学选择性和区域选择性极为吻合。有趣的是,这种化学选择性不能通过评估在TFB 3的C4,C5,C6和O7中心计算的亲电子Parr函数来合理化。在C6-O7化学选择性反应通道中涉及的最优选的过渡态结构TS 1上计算得出的整体电子密度转移值0.31 e证明了这种伪双自由基类型(pdr式)32CA反应具有显着的极性特征。
更新日期:2019-11-19
中文翻译:

探索三氟甲基取代基对硫代羰基S-甲烷与[α,β-不饱和酮] [3 + 2]环加成反应的化学选择性和区域选择性的影响
根据分子电子密度理论在DFT-B3LYP /上研究了硫代羰基内酯(TCY 2)与缺电子的烯酮(TFB 3)在四氢呋喃(THF)之间的[3 + 2]环加成反应(32CA)。6‐31G(d)的计算级别,以探讨能量和选择性。在与TFB 3中的CC和CO化学选择性相关的四个竞争性反应路径中研究了该反应。对基于密度泛函理论的反应性指数进行的分析显示,TCY 2是强亲核试剂,而TFB 3也是强亲电试剂。尽管TFB 3的C4-C5和C6-O7双键都可能参与32CA对TCY 2的反应,计算出的相对吉布斯自由能显然证明,以完全区域选择性的方式参与C6-O7完全优于C4-C5区域,这与实验观察到的化学选择性和区域选择性极为吻合。有趣的是,这种化学选择性不能通过评估在TFB 3的C4,C5,C6和O7中心计算的亲电子Parr函数来合理化。在C6-O7化学选择性反应通道中涉及的最优选的过渡态结构TS 1上计算得出的整体电子密度转移值0.31 e证明了这种伪双自由基类型(pdr式)32CA反应具有显着的极性特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号