European Journal of Human Genetics ( IF 3.7 ) Pub Date : 2019-11-19 , DOI: 10.1038/s41431-019-0540-0 Carin A T C Lunenburg 1 , Cathelijne H van der Wouden 2 , Marga Nijenhuis 3 , Mandy H Crommentuijn-van Rhenen 3 , Nienke J de Boer-Veger 4 , Anne Marie Buunk 5 , Elisa J F Houwink 6 , Hans Mulder 7 , Gerard A Rongen 8, 9 , Ron H N van Schaik 10 , Jan van der Weide 11 , Bob Wilffert 12, 13 , Vera H M Deneer 14 , Jesse J Swen 2 , Henk-Jan Guchelaar 2
|
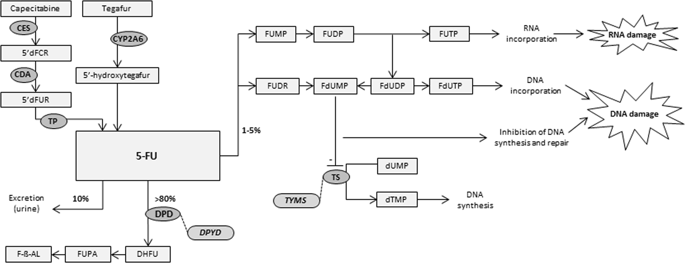
Despite advances in the field of pharmacogenetics (PGx), clinical acceptance has remained limited. The Dutch Pharmacogenetics Working Group (DPWG) aims to facilitate PGx implementation by developing evidence-based pharmacogenetics guidelines to optimize pharmacotherapy. This guideline describes the starting dose optimization of three anti-cancer drugs (fluoropyrimidines: 5-fluorouracil, capecitabine and tegafur) to decrease the risk of severe, potentially fatal, toxicity (such as diarrhoea, hand-foot syndrome, mucositis or myelosuppression). Dihydropyrimidine dehydrogenase (DPD, encoded by the DPYD gene) enzyme deficiency increases risk of fluoropyrimidine-induced toxicity. The DPYD-gene activity score, determined by four DPYD variants, predicts DPD activity and can be used to optimize an individual’s starting dose. The gene activity score ranges from 0 (no DPD activity) to 2 (normal DPD activity). In case it is not possible to calculate the gene activity score based on DPYD genotype, we recommend to determine the DPD activity and adjust the initial dose based on available data. For patients initiating 5-fluorouracil or capecitabine: subjects with a gene activity score of 0 are recommended to avoid systemic and cutaneous 5-fluorouracil or capecitabine; subjects with a gene activity score of 1 or 1.5 are recommended to initiate therapy with 50% the standard dose of 5-fluorouracil or capecitabine. For subjects initiating tegafur: subjects with a gene activity score of 0, 1 or 1.5 are recommended to avoid tegafur. Subjects with a gene activity score of 2 (reference) should receive a standard dose. Based on the DPWG clinical implication score, DPYD genotyping is considered “essential”, therefore directing DPYD testing prior to initiating fluoropyrimidines.
中文翻译:

DPYD 和氟嘧啶的基因-药物相互作用的荷兰药物遗传学工作组 (DPWG) 指南。
尽管药物遗传学(PGx)领域取得了进展,但临床接受度仍然有限。荷兰药物遗传学工作组 (DPWG) 旨在通过制定基于证据的药物遗传学指南来优化药物治疗,从而促进 PGx 的实施。本指南描述了三种抗癌药物(氟嘧啶类:5-氟尿嘧啶、卡培他滨和替加氟)的起始剂量优化,以降低严重、潜在致命的毒性(如腹泻、手足综合征、粘膜炎或骨髓抑制)的风险。二氢嘧啶脱氢酶(DPD,由DPYD基因编码)酶缺乏会增加氟嘧啶诱导毒性的风险。DPYD-基因活性评分,由四个DPYD确定变体,预测 DPD 活性,并可用于优化个体的起始剂量。基因活性评分范围从 0(无 DPD 活性)到 2(正常 DPD 活性)。如果无法根据DPYD计算基因活性分数基因型,我们建议确定 DPD 活性并根据可用数据调整初始剂量。对于开始使用 5-氟尿嘧啶或卡培他滨的患者:建议基因活性评分为 0 的受试者避免全身和皮肤使用 5-氟尿嘧啶或卡培他滨;建议基因活性评分为 1 或 1.5 的受试者开始使用 50% 标准剂量的 5-氟尿嘧啶或卡培他滨进行治疗。对于开始使用替加氟的受试者:建议基因活性评分为 0、1 或 1.5 的受试者避免使用替加氟。基因活性评分为 2(参考)的受试者应接受标准剂量。根据 DPWG 临床意义评分,DPYD基因分型被认为是“必要的”,因此在开始使用氟嘧啶之前指导DPYD检测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号