Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Ubrogepant vs Placebo on Pain and the Most Bothersome Associated Symptom in the Acute Treatment of Migraine
JAMA ( IF 63.1 ) Pub Date : 2019-11-19 , DOI: 10.1001/jama.2019.16711 Richard B Lipton 1 , David W Dodick 2 , Jessica Ailani 3 , Kaifeng Lu 4 , Michelle Finnegan 4 , Armin Szegedi 4 , Joel M Trugman 4
JAMA ( IF 63.1 ) Pub Date : 2019-11-19 , DOI: 10.1001/jama.2019.16711 Richard B Lipton 1 , David W Dodick 2 , Jessica Ailani 3 , Kaifeng Lu 4 , Michelle Finnegan 4 , Armin Szegedi 4 , Joel M Trugman 4
Affiliation
|
Importance
Ubrogepant is an oral calcitonin gene-related peptide receptor antagonist under investigation for acute treatment of migraine. Objective
To evaluate the efficacy and tolerability of ubrogepant compared with placebo for acute treatment of a single migraine attack. Design, Setting, and Participants
Phase 3, multicenter, randomized, double-blind, placebo-controlled, single-attack, clinical trial (ACHIEVE II) conducted in the United States (99 primary care and research clinics; August 26, 2016-February 26, 2018). Participants were adults with migraine with or without aura experiencing 2 to 8 migraine attacks per month. Interventions
Ubrogepant 50 mg (n = 562), ubrogepant 25 mg (n = 561), or placebo (n = 563) for a migraine attack of moderate or severe pain intensity. Main Outcomes and Measures
Co-primary efficacy outcomes were pain freedom and absence of the participant-designated most bothersome migraine-associated symptom (among photophobia, phonophobia, and nausea) at 2 hours after taking the medication. Results
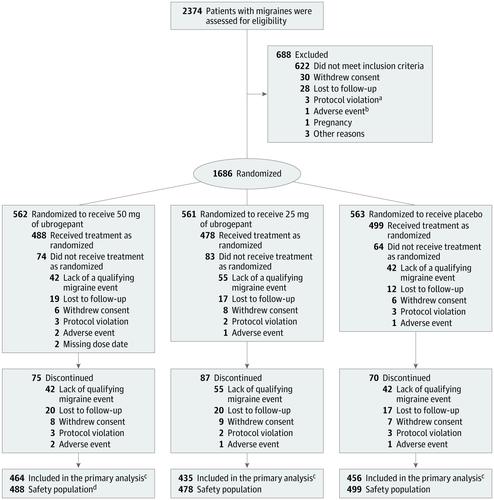
Among 1686 randomized participants, 1465 received study treatment (safety population; mean age, 41.5 years; 90% female); 1355 of 1465 (92.5%) were evaluable for efficacy. Pain freedom at 2 hours was reported by 101 of 464 participants (21.8%) in the ubrogepant 50-mg group, 90 of 435 (20.7%) in the ubrogepant 25-mg group, and 65 of 456 (14.3%) in the placebo group (absolute difference for 50 mg vs placebo, 7.5%; 95% CI, 2.6%-12.5%; P = .01; 25 mg vs placebo, 6.4%; 95% CI, 1.5%-11.5%; P = .03). Absence of the most bothersome associated symptom at 2 hours was reported by 180 of 463 participants (38.9%) in the ubrogepant 50-mg group, 148 of 434 (34.1%) in the ubrogepant 25-mg group, and 125 of 456 (27.4%) in the placebo group (absolute difference for 50 mg vs placebo, 11.5%; 95% CI, 5.4%-17.5%; P = .01; 25 mg vs placebo, 6.7%; 95% CI, 0.6%-12.7%; P = .07). The most common adverse events within 48 hours of any dose were nausea (50 mg, 10 of 488 [2.0%]; 25 mg, 12 of 478 [2.5%]; and placebo, 10 of 499 [2.0%]) and dizziness (50 mg, 7 of 488 [1.4%]; 25 mg, 10 of 478 [2.1%]; placebo, 8 of 499 [1.6%]). Conclusions and Relevance
Among adults with migraine, acute treatment with ubrogepant compared with placebo led to significantly greater rates of pain freedom at 2 hours with 50-mg and 25-mg doses, and absence of the most bothersome migraine-associated symptom at 2 hours only with the 50-mg dose. Further research is needed to assess the effectiveness of ubrogepant against other acute treatments for migraine and to evaluate the long-term safety of ubrogepant among unselected patient populations. Trial Registration
ClinicalTrials.gov Identifier: NCT02867709.
中文翻译:

Ubrogepant 与安慰剂对偏头痛急性治疗中疼痛和最烦人的相关症状的影响
重要性 Ubrogepant 是一种口服降钙素基因相关肽受体拮抗剂,正在研究用于偏头痛的急性治疗。目的 评估 ubrogepant 与安慰剂相比对单次偏头痛发作的急性治疗的疗效和耐受性。设计、设置和参与者 在美国(99 个初级保健和研究诊所;2016 年 8 月 26 日至 2 月)进行的第 3 阶段、多中心、随机、双盲、安慰剂对照、单次攻击、临床试验 (ACHIEVE II) 26, 2018)。参与者是有或没有先兆的偏头痛成年人,每月经历 2 到 8 次偏头痛发作。干预措施 Ubrogepant 50 mg (n = 562)、ubrogepant 25 mg (n = 561) 或安慰剂 (n = 563) 用于中度或重度疼痛强度的偏头痛发作。主要结果和措施 共同主要疗效结果是在服药 2 小时后无疼痛和参与者指定的最烦人的偏头痛相关症状(包括畏光、畏声和恶心)消失。结果 在 1686 名随机参与者中,1465 名接受了研究治疗(安全人群;平均年龄,41.5 岁;90% 为女性);1465 份中的 1355 份 (92.5%) 可评估疗效。ubrogepant 50 毫克组 464 名参与者中的 101 名 (21.8%)、ubrogepant 25 毫克组 435 名参与者中的 90 名 (20.7%) 和安慰剂组 456 名参与者中的 65 名 (14.3%) 报告了 2 小时无疼痛组(50 mg 与安慰剂的绝对差异,7.5%;95% CI,2.6%-12.5%;P = .01;25 mg 与安慰剂,6.4%;95% CI,1.5%-11.5%;P = .03 )。463 名参与者中有 180 人报告在 2 小时内没有最烦人的相关症状 (38. 9%) 在 ubrogepant 50-mg 组中,148 个在 ubrogepant 25-mg 组中的 434 个 (34.1%) 和安慰剂组中的 456 个 (27.4%) 中的 125 个(50 mg 与安慰剂的绝对差异,11.5%; 95% CI,5.4%-17.5%;P = .01;25 mg 对比安慰剂,6.7%;95% CI,0.6%-12.7%;P = .07)。任何剂量后 48 小时内最常见的不良事件是恶心(50 毫克,488 次中的 10 次 [2.0%];25 毫克,478 次中的 12 次 [2.5%];和安慰剂,499 次中的 10 次 [2.0%])和头晕( 50 毫克,488 人中有 7 人 [1.4%];25 毫克,478 人中有 10 人 [2.1%];安慰剂,499 人中有 8 人 [1.6%])。结论和相关性 在患有偏头痛的成人中,与安慰剂相比,ubrogepant 急性治疗在 2 小时时显着提高了 50 毫克和 25 毫克剂量的无痛率,并且仅在 2 小时时没有最令人烦恼的偏头痛相关症状剂量为 50 毫克。需要进一步的研究来评估 ubrogepant 对其他偏头痛急性治疗的有效性,并评估 ubrogepant 在未选择的患者人群中的长期安全性。试验注册 ClinicalTrials.gov 标识符:NCT02867709。
更新日期:2019-11-19
中文翻译:

Ubrogepant 与安慰剂对偏头痛急性治疗中疼痛和最烦人的相关症状的影响
重要性 Ubrogepant 是一种口服降钙素基因相关肽受体拮抗剂,正在研究用于偏头痛的急性治疗。目的 评估 ubrogepant 与安慰剂相比对单次偏头痛发作的急性治疗的疗效和耐受性。设计、设置和参与者 在美国(99 个初级保健和研究诊所;2016 年 8 月 26 日至 2 月)进行的第 3 阶段、多中心、随机、双盲、安慰剂对照、单次攻击、临床试验 (ACHIEVE II) 26, 2018)。参与者是有或没有先兆的偏头痛成年人,每月经历 2 到 8 次偏头痛发作。干预措施 Ubrogepant 50 mg (n = 562)、ubrogepant 25 mg (n = 561) 或安慰剂 (n = 563) 用于中度或重度疼痛强度的偏头痛发作。主要结果和措施 共同主要疗效结果是在服药 2 小时后无疼痛和参与者指定的最烦人的偏头痛相关症状(包括畏光、畏声和恶心)消失。结果 在 1686 名随机参与者中,1465 名接受了研究治疗(安全人群;平均年龄,41.5 岁;90% 为女性);1465 份中的 1355 份 (92.5%) 可评估疗效。ubrogepant 50 毫克组 464 名参与者中的 101 名 (21.8%)、ubrogepant 25 毫克组 435 名参与者中的 90 名 (20.7%) 和安慰剂组 456 名参与者中的 65 名 (14.3%) 报告了 2 小时无疼痛组(50 mg 与安慰剂的绝对差异,7.5%;95% CI,2.6%-12.5%;P = .01;25 mg 与安慰剂,6.4%;95% CI,1.5%-11.5%;P = .03 )。463 名参与者中有 180 人报告在 2 小时内没有最烦人的相关症状 (38. 9%) 在 ubrogepant 50-mg 组中,148 个在 ubrogepant 25-mg 组中的 434 个 (34.1%) 和安慰剂组中的 456 个 (27.4%) 中的 125 个(50 mg 与安慰剂的绝对差异,11.5%; 95% CI,5.4%-17.5%;P = .01;25 mg 对比安慰剂,6.7%;95% CI,0.6%-12.7%;P = .07)。任何剂量后 48 小时内最常见的不良事件是恶心(50 毫克,488 次中的 10 次 [2.0%];25 毫克,478 次中的 12 次 [2.5%];和安慰剂,499 次中的 10 次 [2.0%])和头晕( 50 毫克,488 人中有 7 人 [1.4%];25 毫克,478 人中有 10 人 [2.1%];安慰剂,499 人中有 8 人 [1.6%])。结论和相关性 在患有偏头痛的成人中,与安慰剂相比,ubrogepant 急性治疗在 2 小时时显着提高了 50 毫克和 25 毫克剂量的无痛率,并且仅在 2 小时时没有最令人烦恼的偏头痛相关症状剂量为 50 毫克。需要进一步的研究来评估 ubrogepant 对其他偏头痛急性治疗的有效性,并评估 ubrogepant 在未选择的患者人群中的长期安全性。试验注册 ClinicalTrials.gov 标识符:NCT02867709。











































 京公网安备 11010802027423号
京公网安备 11010802027423号