当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Structural Restraints from Cross-Linking Mass Spectrometry in Human Mitochondria.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jproteome.9b00541 Petra S J Ryl 1 , Michael Bohlke-Schneider 1 , Swantje Lenz 1 , Lutz Fischer 1, 2 , Lisa Budzinski 1 , Marchel Stuiver 1 , Marta M L Mendes 1 , Ludwig Sinn 1 , Francis J O'Reilly 1 , Juri Rappsilber 1, 2
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jproteome.9b00541 Petra S J Ryl 1 , Michael Bohlke-Schneider 1 , Swantje Lenz 1 , Lutz Fischer 1, 2 , Lisa Budzinski 1 , Marchel Stuiver 1 , Marta M L Mendes 1 , Ludwig Sinn 1 , Francis J O'Reilly 1 , Juri Rappsilber 1, 2
Affiliation
|
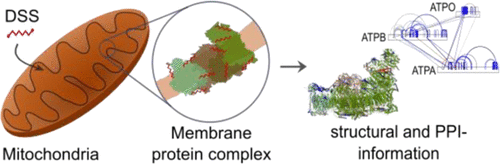
The field of structural biology is increasingly focusing on studying proteins in situ, i.e., in their greater biological context. Cross-linking mass spectrometry (CLMS) is contributing to this effort, typically through the use of mass spectrometry (MS)-cleavable cross-linkers. Here, we apply the popular noncleavable cross-linker disuccinimidyl suberate (DSS) to human mitochondria and identify 5518 distance restraints between protein residues. Each distance restraint on proteins or their interactions provides structural information within mitochondria. Comparing these restraints to protein data bank (PDB)-deposited structures and comparative models reveals novel protein conformations. Our data suggest, among others, substrates and protein flexibility of mitochondrial heat shock proteins. Through this study, we bring forward two central points for the progression of CLMS towards large-scale in situ structural biology: First, clustered conflicts of cross-link data reveal in situ protein conformation states in contrast to error-rich individual conflicts. Second, noncleavable cross-linkers are compatible with proteome-wide studies.
中文翻译:

人体线粒体中交联质谱的原位结构限制。
结构生物学领域越来越关注于原位研究蛋白质,即在更大的生物学范围内研究蛋白质。交联质谱(CLMS)通常通过使用质谱(MS)可裂解的交联剂来促进这一努力。在这里,我们将流行的不可裂解的交联剂二琥珀酰亚胺基辛二酸酯(DSS)应用于人类线粒体,并确定蛋白质残基之间的5518距离限制。对蛋白质或其相互作用的每种距离限制都可在线粒体内提供结构信息。将这些限制条件与蛋白质数据库(PDB)沉积的结构和比较模型进行比较,发现了新颖的蛋白质构象。我们的数据显示了线粒体热休克蛋白的底物和蛋白柔韧性。通过这项研究,我们为CLMS向大规模原位结构生物学的发展提出了两个中心点:首先,与错综复杂的单个冲突相比,交叉链接数据的聚类冲突揭示了原位蛋白的构象状态。其次,不可裂解的交联剂与蛋白质组学研究兼容。
更新日期:2019-12-19
中文翻译:

人体线粒体中交联质谱的原位结构限制。
结构生物学领域越来越关注于原位研究蛋白质,即在更大的生物学范围内研究蛋白质。交联质谱(CLMS)通常通过使用质谱(MS)可裂解的交联剂来促进这一努力。在这里,我们将流行的不可裂解的交联剂二琥珀酰亚胺基辛二酸酯(DSS)应用于人类线粒体,并确定蛋白质残基之间的5518距离限制。对蛋白质或其相互作用的每种距离限制都可在线粒体内提供结构信息。将这些限制条件与蛋白质数据库(PDB)沉积的结构和比较模型进行比较,发现了新颖的蛋白质构象。我们的数据显示了线粒体热休克蛋白的底物和蛋白柔韧性。通过这项研究,我们为CLMS向大规模原位结构生物学的发展提出了两个中心点:首先,与错综复杂的单个冲突相比,交叉链接数据的聚类冲突揭示了原位蛋白的构象状态。其次,不可裂解的交联剂与蛋白质组学研究兼容。











































 京公网安备 11010802027423号
京公网安备 11010802027423号