当前位置:
X-MOL 学术
›
Toxicol. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of the direct peptide reactivity assay (DPRA) to inorganic compounds: a case study of platinum species
Toxicology Research ( IF 2.2 ) Pub Date : 2019-11-20 , DOI: 10.1039/c9tx00242a Jocelyn D. C. Hemming 1 , Mark Hosford 2 , Martin M. Shafer 1, 3
Toxicology Research ( IF 2.2 ) Pub Date : 2019-11-20 , DOI: 10.1039/c9tx00242a Jocelyn D. C. Hemming 1 , Mark Hosford 2 , Martin M. Shafer 1, 3
Affiliation
|
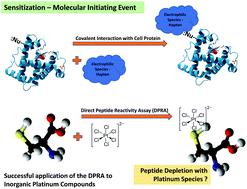
The in chemico Direct Peptide Reactivity Assay (DPRA) was developed as a non-animal, relatively high throughput, screening tool for skin sensitization potential. Although the Adverse Outcome Pathway (AOP) for respiratory sensitization remains to be fully elucidated, it is recognized that the molecular initiation event for both skin and respiratory sensitization to low molecular weight chemicals involves haptenation with proteins. The DPRA examines the reactivity of a test compound to two model peptides (containing either cysteine or lysine) and consequently is able to screen for both skin and respiratory sensitization potential. The DPRA was primarily developed for and validated with organic compounds and assessment of the applicability of the assay to metal compounds has received only limited attention. This paper reports the successful application of the DPRA to a series of platinum compounds, including hexachloroplatinate and tetrachloroplatinate salts, which are some of the most potent chemical respiratory sensitizers known. Eleven platinum compounds were evaluated using the DPRA protocol as detailed by Lalko et al., with only minor modification. Two palladium compounds with structures similar to that of the platinum species studied and cobalt chloride were additionally tested for comparison. The hexachloroplatinate and tetrachloroplatinate salts showed exceptionally high reactivity with the cysteine peptide (EC15 values of 1.4 and 14 μM, respectively). However, for platinum compounds (e.g. hydrogen hexahydroxyplatinate and tetraammineplatinum) where clinical and epidemiological evidence indicates limited sensitization potential, the cysteine DPRA showed only minor or no reactivity (EC15 values of 24 600 and >30 000 μM, respectively). The outcomes of the lysine peptide assays were less robust and where EC15 was measurable, values were substantially higher than the corresponding results from the cysteine assay. This work supports the value of in chemico peptide reactivity as a metric for assessment of platinum sensitization potential and therefore in screening of new platinum compounds for low or absent sensitization potential. Additional studies are required to determine whether the DPRA may be successfully applied to other metals. We provide details on method modifications and precautions important to the success of the DPRA in the assessment of metal reactivity.
中文翻译:

直接肽反应性测定法(DPRA)在无机化合物中的应用:以铂族为例
将在CHEMICO直接肽反应性测定法(DPRA)被开发为用于皮肤致敏潜力的非动物性,相对高通量的筛选工具。尽管呼吸致敏的不良结果途径(AOP)仍有待充分阐明,但人们认识到,皮肤和呼吸致低分子量化学物的分子引发事件都涉及蛋白质的半抗原化。DPRA检查测试化合物对两种模型肽(含有半胱氨酸或赖氨酸)的反应性,因此能够筛选出皮肤和呼吸道致敏性。DPRA最初是为有机化合物开发并经过有机化合物验证的,该方法对金属化合物的适用性评估仅受到了有限的关注。本文报道了DPRA在一系列铂化合物(包括六氯铂酸盐和四氯铂盐)中的成功应用,这些化合物是已知的最有效的化学呼吸敏化剂。如Lalko所述,使用DPRA方案评估了11种铂化合物等。,只需进行少量修改。另外测试了两种结构与所研究的铂物种相似的钯化合物和氯化钴进行比较。六氯铂盐和四氯铂盐显示出与半胱氨酸肽的异常高的反应性(EC 15值分别为1.4和14μM)。但是,对于临床和流行病学证据表明敏化潜能有限的铂化合物(例如,六羟基铂酸氢盐和四氨合铂),半胱氨酸DPRA的反应性很小或没有(EC 15值分别为24 600和> 30 000μM )。赖氨酸肽测定的结果不那么稳健,在EC 15的情况下可以测量,其值大大高于半胱氨酸测定的相应结果。这项工作支持化学肽反应性的价值,作为评估铂敏化潜能的指标,因此可用于筛选新的铂化合物的敏化潜能低或不存在。需要进行其他研究以确定DPRA是否可以成功应用于其他金属。我们提供了有关在金属反应性评估中对DPRA成功至关重要的方法修改和预防措施的详细信息。
更新日期:2019-11-20
中文翻译:

直接肽反应性测定法(DPRA)在无机化合物中的应用:以铂族为例
将在CHEMICO直接肽反应性测定法(DPRA)被开发为用于皮肤致敏潜力的非动物性,相对高通量的筛选工具。尽管呼吸致敏的不良结果途径(AOP)仍有待充分阐明,但人们认识到,皮肤和呼吸致低分子量化学物的分子引发事件都涉及蛋白质的半抗原化。DPRA检查测试化合物对两种模型肽(含有半胱氨酸或赖氨酸)的反应性,因此能够筛选出皮肤和呼吸道致敏性。DPRA最初是为有机化合物开发并经过有机化合物验证的,该方法对金属化合物的适用性评估仅受到了有限的关注。本文报道了DPRA在一系列铂化合物(包括六氯铂酸盐和四氯铂盐)中的成功应用,这些化合物是已知的最有效的化学呼吸敏化剂。如Lalko所述,使用DPRA方案评估了11种铂化合物等。,只需进行少量修改。另外测试了两种结构与所研究的铂物种相似的钯化合物和氯化钴进行比较。六氯铂盐和四氯铂盐显示出与半胱氨酸肽的异常高的反应性(EC 15值分别为1.4和14μM)。但是,对于临床和流行病学证据表明敏化潜能有限的铂化合物(例如,六羟基铂酸氢盐和四氨合铂),半胱氨酸DPRA的反应性很小或没有(EC 15值分别为24 600和> 30 000μM )。赖氨酸肽测定的结果不那么稳健,在EC 15的情况下可以测量,其值大大高于半胱氨酸测定的相应结果。这项工作支持化学肽反应性的价值,作为评估铂敏化潜能的指标,因此可用于筛选新的铂化合物的敏化潜能低或不存在。需要进行其他研究以确定DPRA是否可以成功应用于其他金属。我们提供了有关在金属反应性评估中对DPRA成功至关重要的方法修改和预防措施的详细信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号