当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Neutral, cationic and anionic organonickel and -palladium complexes supported by iminophosphine/phosphinoenaminato ligands.
Dalton Transactions ( IF 4 ) Pub Date : 2019-11-19 , DOI: 10.1039/c9dt04062e Tomás G Santiago 1 , Carmen Urbaneja , Eleuterio Álvarez , Elena Ávila , Pilar Palma , Juan Cámpora
Dalton Transactions ( IF 4 ) Pub Date : 2019-11-19 , DOI: 10.1039/c9dt04062e Tomás G Santiago 1 , Carmen Urbaneja , Eleuterio Álvarez , Elena Ávila , Pilar Palma , Juan Cámpora
Affiliation
|
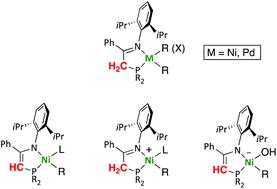
We report a series of organometallic nickel and palladium complexes containing iminophosphine ligands R2PCH2C(Ph) = N-Dipp (Dipp = 2,6-diisopropylphenyl; R = iPr, La; R = Ph, Lb; and R = o-C6H4OMe, Lc), synthesized by ligand exchange or oxidative addition reactions, and we investigate the capacity of such ligands to undergo reversible deprotonation to the corresponding phosphinoenaminato species. In the attempted ligand exchange reaction of the nickel bis(trimethylsilyl)methyl precursor [Ni(CH2SiMe3)2Py2] with Lb, the iminophosphine acts as a weak acid rather than a neutral ligand, cleaving one of the Ni–C bonds, to afford the phosphinoenaminato complex [Ni(CH2SiMe3)(L′b)(Py)] (L′b = conjugate base of Lb). We disclose a general method for the syntheses of complexes [Ni(CH2SiMe3)(L)(Py)]+ (L = La, Lb or Lc), and demonstrate that iminophosphine deprotonation is a general feature and occurs reversibly in the coordination sphere of the metal. By studying proton exchange reactions of the cation [Ni(CH2SiMe3)(Lb)(Py)]+ with bases of different strength we show that the conjugate phosphinoenaminato ligand in [Ni(CH2SiMe3)(L′b)(Py)] is a base with strength comparable to DBU in THF. The acyl group in the functionalized aryl complex [Ni(p-C6H4COCH3)(Br)(La)] does not interfere in the iminophosphine deprotonation with NaH. The latter reaction affords the unusual anionic hydroxide species [Ni(p-C6H4COCH3)(OH)(L′a)]−Na+, which was isolated and fully characterized.
中文翻译:

亚氨基膦/膦氨基甲酸酯配体支持的中性,阳离子和阴离子有机镍和-钯配合物。
我们报告了一系列含有亚氨基膦配体R 2 PCH 2 C(Ph)= N-Dipp(Dipp = 2,6-diisopropylphenyl; R = iPr,La ; R = Ph,Lb ;和R = o的有机金属镍和钯配合物-C 6 H 4 OMe,Lc),通过配体交换或氧化加成反应合成,我们研究了这些配体经历可逆去质子化反应生成相应膦酰氨基的能力。在双(三甲基甲硅烷基)甲基镍前体[Ni(CH 2 SiMe 3)2 Py 2 ]与Lb的配体交换反应中,亚氨基膦作为一种弱酸而不是中性配体,裂解一个Ni–C键,以提供膦氨基甲酸酯络合物[Ni(CH 2 SiMe 3)(L'b)(Py)](L'b = Lb的共轭碱基)。我们公开了一种合成配合物[Ni(CH 2 SiMe 3)(L)(Py)] +(L = La,Lb或Lc)的通用方法,并证明亚氨基膦去质子化是一个通用特征并且可逆地发生在金属的协调范围。通过研究阳离子[Ni(CH 2SiMe 3)(Lb)(Py)] +具有不同强度的碱,我们显示[Ni(CH 2 SiMe 3)(L'b)(Py)]中的共轭膦酰氨基配体是强度与DBU中的DBU相当的碱四氢呋喃。官能化的芳基配合物[Ni(p -C 6 H 4 COCH 3)(Br)(La)]中的酰基不会干扰亚氨基NaH的亚氨基膦去质子化。后一反应提供了不同寻常的阴离子氢氧化物[Ni(p -C 6 H 4 COCH 3)(OH)(L'a)] -Na +,已被分离并充分表征。
更新日期:2019-11-19
中文翻译:

亚氨基膦/膦氨基甲酸酯配体支持的中性,阳离子和阴离子有机镍和-钯配合物。
我们报告了一系列含有亚氨基膦配体R 2 PCH 2 C(Ph)= N-Dipp(Dipp = 2,6-diisopropylphenyl; R = iPr,La ; R = Ph,Lb ;和R = o的有机金属镍和钯配合物-C 6 H 4 OMe,Lc),通过配体交换或氧化加成反应合成,我们研究了这些配体经历可逆去质子化反应生成相应膦酰氨基的能力。在双(三甲基甲硅烷基)甲基镍前体[Ni(CH 2 SiMe 3)2 Py 2 ]与Lb的配体交换反应中,亚氨基膦作为一种弱酸而不是中性配体,裂解一个Ni–C键,以提供膦氨基甲酸酯络合物[Ni(CH 2 SiMe 3)(L'b)(Py)](L'b = Lb的共轭碱基)。我们公开了一种合成配合物[Ni(CH 2 SiMe 3)(L)(Py)] +(L = La,Lb或Lc)的通用方法,并证明亚氨基膦去质子化是一个通用特征并且可逆地发生在金属的协调范围。通过研究阳离子[Ni(CH 2SiMe 3)(Lb)(Py)] +具有不同强度的碱,我们显示[Ni(CH 2 SiMe 3)(L'b)(Py)]中的共轭膦酰氨基配体是强度与DBU中的DBU相当的碱四氢呋喃。官能化的芳基配合物[Ni(p -C 6 H 4 COCH 3)(Br)(La)]中的酰基不会干扰亚氨基NaH的亚氨基膦去质子化。后一反应提供了不同寻常的阴离子氢氧化物[Ni(p -C 6 H 4 COCH 3)(OH)(L'a)] -Na +,已被分离并充分表征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号