当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective electrosynthesis of tetra- and hexa-functionalized [60]fullerene derivatives with unprecedented addition patterns
Chemical Science ( IF 7.6 ) Pub Date : 2019-11-20 , DOI: 10.1039/c9sc02131k Kai-Qing Liu 1 , Jun-Jie Wang 1 , Xing-Xing Yan 1 , Chuang Niu 1 , Guan-Wu Wang 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2019-11-20 , DOI: 10.1039/c9sc02131k Kai-Qing Liu 1 , Jun-Jie Wang 1 , Xing-Xing Yan 1 , Chuang Niu 1 , Guan-Wu Wang 1, 2
Affiliation
|
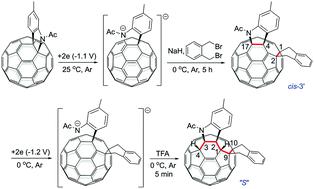
The efficient and regioselective electrosynthesis of tetra- and hexa-functionalized [60]fullerene derivatives with unprecedented addition patterns has been achieved. The tetra-functionalized [60]fullerene derivative with an intriguing 1,2,4,17-addition pattern is regioselectively obtained by cyclization reaction of the dianionic species generated electrochemically from a [60]fulleroindoline with 1,2-bis(bromomethyl)benzene at 0 °C, and can be converted to the more stable 1,2,3,4-adduct at 25 °C. Furthermore, the hexa-functionalized [60]fullerene derivative with the 1,2,3,4,9,10-addition pattern displaying a unique “S”-shaped configuration can be synthesized by protonation of the electrochemically generated dianion of the obtained tetra-functionalized 1,2,4,17-adduct. The structures of the tetra- and hexa-functionalized products have been determined by spectroscopic data and single-crystal X-ray analysis.
中文翻译:

具有前所未有的加成模式的四和六官能化[60]富勒烯衍生物的区域选择性电合成
已经实现了具有前所未有的加成模式的四和六官能化[60]富勒烯衍生物的高效和区域选择性电合成。通过[60]富勒二氢吲哚与1,2-双(溴甲基)苯电化学生成的双阴离子物质的环化反应,区域选择性地获得了具有有趣的1,2,4,17加成模式的四官能化[60]富勒烯衍生物在0℃下,可以在25℃下转化为更稳定的1,2,3,4-加合物。此外,通过电化学生成的四阴离子的质子化,可以合成具有1,2,3,4,9,10加成模式的六官能化[60]富勒烯衍生物,显示出独特的“ S ”形构型。 -官能化1,2,4,17-加合物。四官能化和六官能化产物的结构已通过光谱数据和单晶 X 射线分析确定。
更新日期:2019-11-20
中文翻译:

具有前所未有的加成模式的四和六官能化[60]富勒烯衍生物的区域选择性电合成
已经实现了具有前所未有的加成模式的四和六官能化[60]富勒烯衍生物的高效和区域选择性电合成。通过[60]富勒二氢吲哚与1,2-双(溴甲基)苯电化学生成的双阴离子物质的环化反应,区域选择性地获得了具有有趣的1,2,4,17加成模式的四官能化[60]富勒烯衍生物在0℃下,可以在25℃下转化为更稳定的1,2,3,4-加合物。此外,通过电化学生成的四阴离子的质子化,可以合成具有1,2,3,4,9,10加成模式的六官能化[60]富勒烯衍生物,显示出独特的“ S ”形构型。 -官能化1,2,4,17-加合物。四官能化和六官能化产物的结构已通过光谱数据和单晶 X 射线分析确定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号