The Pharmacogenomics Journal ( IF 2.9 ) Pub Date : 2019-11-20 , DOI: 10.1038/s41397-019-0118-9 Beatriz Planelles 1, 2 , César Margarit 1, 2 , María-Del-Mar Inda 2 , Pura Ballester 2 , Javier Muriel 2 , Jordi Barrachina 3 , Raquel Ajo 2 , María-Dolores Esteban 4 , Ana M Peiró 1, 2, 5
|
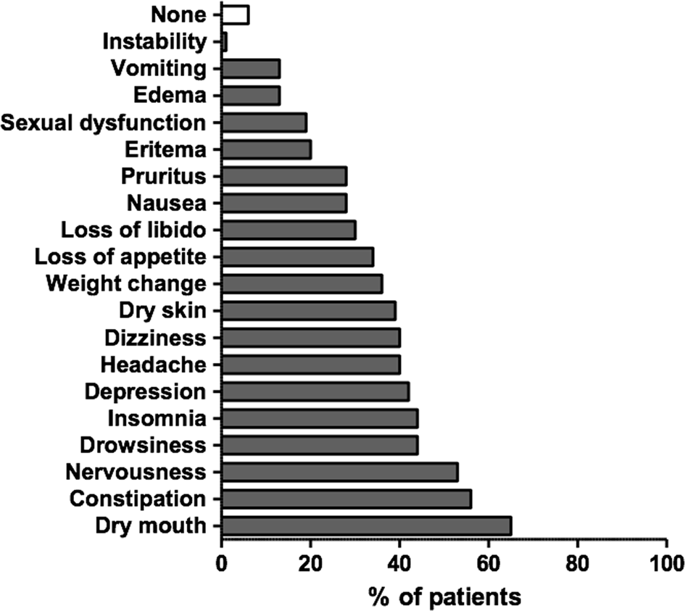
Safety data in chronic non-cancer pain (CNCP) with long-term opioid therapy has been poorly studied and can be differently influenced by gender. Furthermore, pharmacogenetics (PGx) could possibly be used to tailor pain medication based on the individual’s genetic background. The aim was to assess whether PGx applied to a pharmacovigilance system could help to improve a patient’s security profile. A pharmacovigilance data recording system was conducted over 24 months, including genotyping of OPRM1 variants (opioid receptor, A118G) and COMT (enzyme that degrades catecholamines such as norepinephrine, G1947A). Pain intensity (visual analogue scale, VAS), morphine equivalent daily dose (MEDD), adverse events (AEs) and suspected adverse drug reactions (ADRs) were recorded and analysed by gender. The Ethics Committee approved the study and data were analysed with R 3.6.0 software. A total of 748 patients were recruited in the study (67% female, VAS 62 ± 29 mm, MEDD 119 ± 114 mg/day) reporting a median of 6 (3.5–9) AEs/patient. Women presented more nausea, headaches, insomnia, loss of appetite, weight change, depression and dizziness than men. Analysis by genotype demonstrated that PGx influenced the prevalence of vomiting and depression in men, dizziness in women and sexual dysfunction in both. Physicians notified 150 ADRs mostly in females (79%) related to nervous system disorders. PGx applied to a pharmacovigilance recording system provides important information to achieve a better knowledge about AEs in CNCP pharmacological therapy. OPRM1 and COMT polymorphisms were associated with AEs in CNCP patients that differed according to gender.
中文翻译:

慢性疼痛管理中基于性别的差异,药物遗传学和不良事件。
长期使用阿片类药物治疗的慢性非癌性疼痛(CNCP)的安全性数据研究很少,并且可能受到性别的不同影响。此外,根据个体的遗传背景,药物遗传学(PGx)可能会用于定制止痛药。目的是评估将PGx应用于药物警戒系统是否可以帮助改善患者的安全性。药物警戒数据记录系统进行了24个月,包括OPRM1变体(阿片受体,A118G)和COMT的基因分型(降解儿茶酚胺的酶,例如去甲肾上腺素,G1947A)。记录疼痛强度(视觉模拟量表,VAS),吗啡当量日剂量(MEDD),不良事件(AEs)和可疑药物不良反应(ADRs),并按性别进行分析。伦理委员会批准了这项研究,并使用R 3.6.0软件对数据进行了分析。该研究共招募了748名患者(67%为女性,VAS 62±29 mm,MEDD 119±114 mg /天),报告中位数为6(3.5–9)AE /患者。与男性相比,女性表现出更多的恶心,头痛,失眠,食欲不振,体重减轻,抑郁和头晕。通过基因型分析表明,PGx影响男性呕吐和抑郁的患病率,女性头晕和两者的性功能障碍。医生通知了150例ADR,其中大多数是与神经系统疾病有关的女性(占79%)。OPRM1和COMT基因多态性与CNCP患者的AE相关,这取决于性别。









































 京公网安备 11010802027423号
京公网安备 11010802027423号